Chemoprevention Goes Gourmet: Different Flavors of NO-Aspirin
Colorectal cancer (CRC) is a common disease associated with a substantial chance of mortality, largely because many patients present with advanced disease, rendering curative treatment difficult or impossible. In keeping with the old axiom that prevention is better than cure, attention is increasingly focused on the chemoprevention of this disease. The concept of preventing CRC is particularly tantalizing, in part because of the effects of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) on the mortality associated with this disease. After suffering setbacks brought about by problems with the coxib [selective inhibitors of the cyclooxygenase-2 (Cox-2) enzyme] class of drugs, recent work by Gao et al. (1) and Kashfi et al. (2) have infused new optimism into the field, and suggest that certain NSAIDs might have as-yet untapped promise for the prevention of CRC. Their findings provide new insights into the action of aspirin, and emphasize the possibility that the efficacy of aspirin might be further enhanced by modifications to the parent molecule, permitting us to circumvent undesirable gastrointestinal side effects, while still exploiting the benefits of this century-old medicine.
Interest in the chemoprevention of CRC has increased significantly over the past fifteen years, following evidence from epidemiological studies that have shown significantly lower rates of CRC in individuals reporting regular consumption of aspirin and other NSAIDs (3). Since this time, a large number of retrospective and prospective studies have confirmed that regular aspirin users are substantially less likely than nonusers to develop colorectal cancer or its precursor, adenomatous polyps (4, 5). The studies have been remarkably consistent, and virtually all demonstrate a protective, dose-related effect for aspirin. Even stronger evidence has been obtained through randomized trials in which short-term use of aspirin taken in doses of either 81 mg or 325 mg daily reduces the risk for recurrent adenomas in patients who have already had either a CRC or colorectal adenoma (6, 7).
There are two concerns about using these agents in fundamentally healthy people to prevent a disease they are not likely to develop. First, the reduction in cancer incidence associated with the use of these compounds is generally <50% (6), which may or may not be suitable, depending upon each patient’s pre-existing risk factors. Second, NSAIDs routinely cause gastropathy, which may produce dyspepsia, mucosal ulceration, upper gastrointestinal bleeding, and, occasionally, death (8). This controversy prompts the obvious question: how do we avoid the side-effects of aspirin and other NSAIDs, and still retain the beneficial effects?
Several innovative approaches have been employed in the last decade to tackle this issue, one of which was the development coxibs. This significantly reduced the gastropathy, but unfortunately, increased cardiovascular problems in elderly patients on long-term therapy, and attracted front-page attention in the press (9). Another innovative approach to this quandary has been the development of novel gastric-sparing nitric oxide (NO)-donating NSAIDs (NO-NSAIDs), which, for now, hold the greatest promise as agents for cancer prevention.
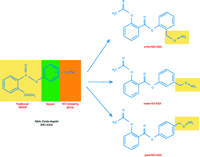
NO is an important mediator of gastric mucosal defense, and the hypothesis that a potentially injurious agent like an NSAID could be prevented by a defensive chaperone led to the development of NO-NSAIDs. Under normal physiological conditions, small amounts of locally generated NO contribute to gastric mucosal defense by influencing prostaglandin synthesis, mucus production, bicarbonate secretion, as well as participating in the regulation of the microcirculation (10). NO-NSAIDs consist of a conventional NSAID to which an NO-donating moiety (typically–ONO2) is covalently bound by a spacer (a short-chain ester linkage) as shown in Figure 1⇓ (11). The rationale behind coupling an NO-releasing moiety to an NSAID was that the NO released would be beneficial to the gastric mucosa by enhancing local defenses, and by preventing pathogenic events that occur as a consequence of the very high local levels of NSAID in the stomach (where the pill begins to dissolve) without abrogating the systemic effects of the drug. Presumably, the locally released NO would prevent the suppression of prostaglandin biosynthesis, reduced gastric mucosal blood flow, increased tumor necrosis factor–α (TNF-α) concentration, and altered leukocyte–endothelial cell adherence. Although these compounds must be subjected to clinical phase 2 and 3 trials, the limited laboratory and clinical data available demonstrate that these compounds are associated with markedly reduced gastrointestinal toxicity, while retaining the anti-inflammatory activities of the parent NSAID (12, 13).
NO-aspirin is a “designer drug” whose two active ingredients have individually enjoyed molecule of the year status: acetylsalicylic acid in 1897, and nitric oxide in 1992 (14). In 1995, it was reported that the nitroxybutylester derivative of aspirin (NCX 4215) was nearly seven times more potent in preventing platelet aggregation without inhibiting gastric prostaglandin production, which prevented mucosal damage in the stomachs of rats (13, 15). More than simple avoidance of side-effects, the NO brought synergy to the pharmacological equation. Fiorucci et al. found that NO-ASA (NCX-4016) produced a platelet inhibitory effect identical to aspirin without any visible (endoscopic) evidence of gastric toxicity in healthy human subjects treated with this compound (16). Might one get similar therapeutic anti-cancer effects with these drugs? This is a more complex issue, but this possibility is supported by laboratory studies evaluating the effect of NO-ASA on the growth inhibition of cultured colon cancer cells, which showed that the IC50 of NO-ASA was several hundred-fold less than parent ASA (17).
The recent publications by Gao et al. (1) and Kashfi et al. (2) embellish the biological possibilities of NO-ASA further. These papers elegantly demonstrate that the positional isomers of NO-ASA (with respect to the position of nitric oxide-donating –ONO2 group at ortho, meta, and para positions) are important determinants of the potency of the compound. The investigators examined whether this positional isomerism affects the biological activity of NO-ASA in cultured HT-29 human colon cancer cells. The ortho- and para-isomers had significantly lower IC50 values and induced apoptosis and the formation of atypical cells when compared to the meta-isomer at seventy-two hours post-administration. These creative observations are consistent with the findings reported by other laboratories (17).
As compelling as these observations may be, skeptics will understandably challenge conclusions drawn from data obtained from a single cell line. From a mechanistic viewpoint, the authors contend that the growth inhibitory effects of this compound may arise from enhanced apoptosis in these cells. It has been suggested that NO promotes apoptosis and growth inhibition of cancer cells by activating the p53 cascade (18). This is a complex issue for HT-29 cells, which have inactivating TP53 mutations and do not have a functional p53 response. Therefore, the induction of programmed cell death in HT-29 cells by NO-ASA is necessarily p53-independent, which underscores that the effects of this compound must also be evaluated in cell models with intact components of the apoptotic signaling cascade.
It is wise to be cautious in the analysis of any data originating from pure in vitro cell models, and it is necessary to validate such findings in animal models. Following this paradigm, the laboratories of Gao and Kashfi extended their work to animal studies, and confirmed that the para-isomer was significantly more effective than the meta-isomer in reducing the tumor burden in the small intestine of the APCmin/+ mouse. Additionally, structure-activity studies of the three NO-ASA isomers revealed that substituting an aliphatic chain for the aromatic spacer, or removing the –ONO2 group, profoundly diminished the ability of NO-ASA to inhibit cell growth, whereas removal of the acetyl group on the ASA moiety did not.
What makes the meta-isomer a less effective compound compared to the ortho and para isomers? The differential behavior of the three positional isomers of NO-ASA, both in vitro and in mice, was clarified by the increased rates of metabolic transformation of the ortho- and para-isomers of NO-ASA by fractions of rat liver and colon and cultured human colon cancer cells.
Despite these compelling implications for the use of NO-ASA in the chemoprevention of CRC, these studies still leave us with several questions. The important issue is whether an agent with broad biologic properties, taken daily for life, might lead to undesirable effects. As has been observed with multiple new drugs, some unsuspected side effects may surface only after long-term evaluation in controlled clinical trials. Toxicity in humans is not always predictable from preclinical work, and the cardiovascular problems associated with COX-2 inhibitors should be a sufficient warning in this regard. Beyond the issue of unpredictable acute adverse events, a key question remains to be answered regarding the long-term use of these agents: will NO-ASA be tolerated by the gastrointestinal tract when administered for decades? Enteric-coated aspirin causes minimal acute mucosal damage when administered for one week (19), but is frequently associated with gastrointestinal complications when used chronically (20).
The anti-cancer effects of aspirin and NSAIDs appear to be rather broad, and evidence of reduced cancer mortality can be found throughout the gastrointestinal tract. How good are the new designer drugs? There are no perfect preventive agents, and the development of breast and prostate cancer are apparently not affected by NSAIDs. Although we can only speculate, many cancers share common pathogenetic pathways (such as TP53 mutations), but there are very substantial differences between cancers. The WNT–β-catenin–APC signaling pathway is more important for gastrointestinal epithelial tumors than for other tumors. Although we can observe anti-neoplastic effects both in the laboratory and in human trials, we do not understand the fundamental, common mechanisms underlying chemoprevention. One particular problem is that human CRCs are quite heterogeneous both at the microgenetic level (i.e., each tumor has its own constellation of mutations) and at the macrogenetic level (i.e., there are at least three separate forms of genomic or epigenetic instability in CRCs) (21). Consider, for the moment, just the APC gene, the tumor suppressor gene thought to be the “gatekeeper” for colorectal neoplasia: it can be inactivated by mutation, deletion, or promoter methylation, or any combinations of these (22, 23). These issues will become particularly important as trials are launched in very high-risk patients with the two well-recognized forms of hereditary CRC: familial adenomatous polyposis (FAP) and Lynch syndrome (also called hereditary nonpolyposis colorectal cancer or HNPCC). These are very different diseases, have mechanistic correlates in sporadic cancers, and may require very different preventive strategies. We have previously reported that aspirin increases DNA mismatch repair protein expression in vitro, however, it does not improve replication fidelity in this model (24, 25). Although the large clinical trials have not yet reached maturity for analysis, the early data suggest that aspirin may not be effective in Lynch syndrome patients. In the case of CRCs associated with hMLH1 promoter hypermethylation, aspirin has been tested neither in vitro nor in vivo. We should be prepared for the possibility that aspirin and its derivates may only be active in preventing certain types of CRCs and that it is too early to know which ones to use.
There are some suggestions that the anti-cancer effects of NO-NSAIDs may not be restricted to CRC cell lines, as they appear to inhibit the growth of cell lines from various types of cancer (26). From a clinical viewpoint, an equally important question relates to the fact that because NO-aspirin inhibits COX-1-dependent platelet function, we should be prepared for the possibility that perturbations in COX-1-linked prostaglandin synthesis in the GI mucosa may not be well tolerated. The persistent, progressive damage that leads to ulcers and their complications in the gut is mainly related to chronic mucosal prostaglandin deficiency. Moreover, we still have only a vague notion of the ideal dose of aspirin against which to compare NO-ASA (27–29).
There are no easy answers to the question as to how NO-aspirin will perform clinically in CRC and other malignancies. However, the findings by Basil Rigas’s group (1, 2) are provocative and force us to think carefully about the actions of these novel compounds. In the interim it may be wise to interpret these results as strong preliminary evidence, serving as an important proof of concept. Further studies will supply answers to the other questions, answers that are necessary before NO-aspirin can be recommended for clinical use. Fortunately, conflicting or supporting data from diverse threads of research are often a very effective push toward scientific progress.
The chemical structures of NO-aspirin and its positional isomers. The NO-aspirin molecule is made of aspirin (acetylsalicylic acid) covalently linked through an aromatic spacer to an NO-releasing moiety (–ONO2). The three positional isomers of NO-aspirin, ortho, meta, and para, reflect the position of –ONO2 group linkage on the aromatic spacer.
- © American Society for Pharmacology and Experimental Theraputics 2005
References

Rick Boland, MD, is a gastroenterologist with an interest in familial colorectal cancer and the mechanistic basis of colorectal cancer. He is currently Chief of Gastroenterology at Baylor University Medical Center, in Dallas, Texas, and Clinical Professor of Internal Medicine at the University of Texas, Southwestern, in Dallas. E-mail rickbo{at}baylorhealth.edu

Christoph Gasche, MD, was a visiting research scholar in the laboratory of Dr Boland from 1999–2001 and has a research interest in inflammatory bowel disease and the mechanisms of carcinogenesis in chronic inflammation. He is currently an Associate Professor of Medicine in the Division of Gastroenterology and Hepatology at the Medical University of Vienna. E-mail christoph.gasche{at}meduniwien.ac.at

Ajay Goel, PhD, is a Senior Scientist in the Baylor Research Institute, and has been a colleague of Dr Boland for the past ten years. His expertise is in the molecular genetics of colorectal cancer, and he has made contributions to our understanding of the mechanisms of genomic instability in cancer. E-mail ajayg{at}baylorhealth.edu