
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Immunodeficiency Is A Tough Nut to CRAC: The Importance of Calcium Flux in T Cell Activation
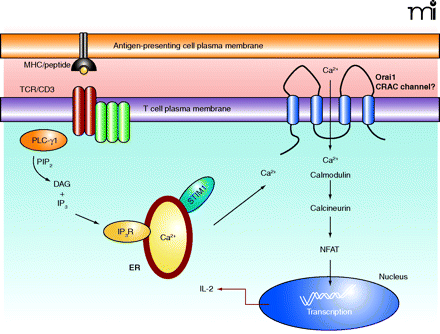
Calcium is essential for the effector processes that occur downstream of T cell receptor activation. T cells recognize antigen in the form of a peptide fragment presented in the context of MHC via the TCR/CD3 complex on the cell surface. (TCR appears as two dark red ovals; CD3 complex appears as three green ovals adjacent to TCR.) Activation results in the phosphorylation of PLC-γ1, leading to the hydrolysis of PIP2, generating DAG and IP3. IP3 causes the release of calcium from stores within the endoplasmic reticulum which is detected by STIM1. This causes the opening of CRAC channels in the plasma membrane and Ca2+ influx leading to NFAT activation, via calmodulin and calreticulin, and the transcription of genes responsible for T cell effector function (i.e., IL-2). TCR, T cell antigen receptor; MHC, major histocompatability complex; PLC-γ1; phospholipase C–γ1; PIP2, phosphatidylinositol bisphosphate; DAG, diacylglycerol; IP3, inositol-3,4,5-trisphosphate; IP3R, inositol trisphosphate receptor; ER, endoplasmic reticulum; NFAT, nuclear factor of activated T cells; CRAC, Ca2+-release-activated Ca2+channel; IL-2, interleukin-2.


