
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Unraveling the Story of NGF-mediated Sensitization of Nociceptive Sensory Neurons: ON or OFF the Trks?
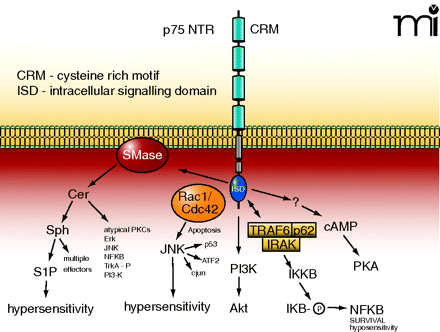
The p75 NTR and its associated intracellular signalling pathways. The p75 NTR is made up of four extracellular cysteine rich motifs (CRM), a single membrane spanning domain, and an intracellular signaling domain (ISD). NGF binding causes the activation of sphingomyelinases (SMase) that lead to the liberation of ceramide (Cer) and subsequently to sphingosine (Sph) and sphingosine 1-phosphate (S1P). Ceramide and sphingosine can activate a multitude of effector pathways. In small-diameter sensory neurons the generation of S1P appears to enhance the firing of action potential through the modulation of a TTX-resistant sodium current and the suppression of a delayed rectifier-like current in sensory neurons. The small G proteins Rac1/Cdc42 associate with the ISD, which results in the activation of JNK. In many cell systems, activation of JNK is critically involved in apoptosis, however, in sensory neurons, activation of JNK gives rise to hypersensitivity. The ISD, through an undefined mechanism, results in the activation of PI3K with downstream activation of Akt. The ISD can associate with the complex formed by TRAF-IRAK-p62 to activate IKKβ, which then phosphorylates IκB and release NF-κB. In sensory neurons, activation of the NF-κB pathway appears to be anti-nociceptive. Lastly, activation of p75 somehow leads to increased levels of intracellular cyclic AMP and activation of PKA. See text for details


