
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Regulation of Receptors and Transporters by Ubiquitination: New Insights into Surprisingly Similar Mechanisms
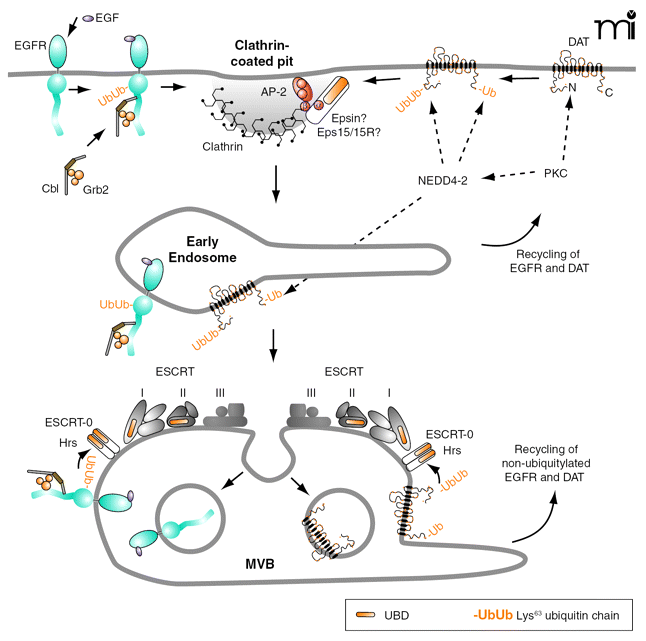
Hypothetic model of endocytosis and endosomal sorting of the EGF receptor and DAT. (Top left) Binding of epidermal growth factor (EGF) to the EGF receptor (EGFR) results in recruitment of the Grb2-Cbl complex and receptor ubiquitination. EGFR is internalized via clathrin-coated pits with participation of Grb2 and Cbl by an unknown mechanism. After fusion of clathrin-coated vesicles with early endosomes (middle), EGFR can either recycle directly back to the plasma membrane or remain in the maturing endosome that acquires ESCRT complexes. Ubiquitinated receptors bind to the UBD of the ESCRT-0 complex, and eventually become trapped in the intralumenal vesicles of MVB (bottom). Non-ubiquitinated receptors can recycle back to the cell surface through the tubular extensions of MVB. (Top right) Activation of protein kinase C (PKC) results in NEDD4-2–mediated ubiquitination of the dopamine transporter (DAT). PKC activation can facilitate NEDD4-2–mediated ubiquitination of DAT either by phosphorylating DAT or DAT-interacting proteins, or by activating NEDD4-2. Ubiquitinated DAT is recruited into clathrin-coated pits by means of interaction with the UBD-containing proteins, such as Eps15/Eps15R and epsin, bound to AP-2 and clathrin in coated pits. PKC-dependent sorting of DAT in early endosomes (middle) and MVB (bottom) is hypothesized to occur by the mechanism similar to that of the EGFR. See text for details.


