
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Cardiac Glycosides as Novel Cancer Therapeutic Agents
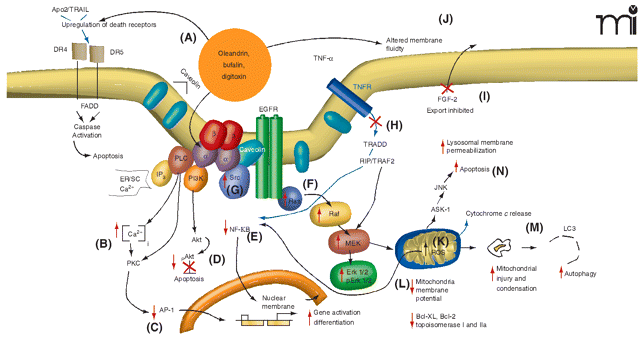
The Na+,K+-ATPase signalosome complex. The binding of selected cardiac glycosides (CGs)––such as oleandrin, bufalin, and digitoxin––to Na+,K+-ATPase results in complex but well-documented changes in cell signaling events. The “signalosome” complex includes the enzyme, Na+,K+-ATPase as well as Src, phosphoinositide-3 kinase (PI3K), and phospholipase C each of which, in turn, sets into action complex signaling events that can result in tumor cell death through either apoptosis or autophagy-related mechanisms. Administration of CGs can (A) increase the (cell surface) expression of death receptors (DR4, DR5) and activate caspase activity; (B) result in increased intracellular calcium concentrations, which, in turn, (C) decreases the expression of transcription factors such as Activator Protein-1 (AP-1). CG treatment can also (D) inhibit activation (i.e., block the phosphorylation) of Akt, which normally blocks apoptosis; (E) inhibit activation of the transcription factor Nuclear Factor-kappaB (NF-κ B); (F) activate the Ras pathway, leading to increase activity of Raf–MAPK pathway; (G) activate Src; (H) inhibit tumor necrosis factor (TNF)-mediated activation of NF-κ B by inhibiting the binding of tumor necrosis factor receptor 1–associated death domain protein (TRADD) to the cellular membrane; (I) inhibit extracellular transport of tumor growth factors, such as fibroblast growth factor-2 (FGF-2); (J) alter membrane fluidity which, in turn, may inhibit Fas-related signaling; (K) lead to the production of reactive oxygen species (ROS) with subsequent injury to mitochondria; (L) produce a decrease in mitochondria membrane potential and a decrease in quantity of anti-apoptotic proteins Bcl-XL and Bcl-2 and topoisomerases I and II; and (M) cause mitochondrial condensation and loss of function, that, in turn, can lead to autophagic processes and cell death (N). Adapted from (30).


