The Outcome of Selenium and Vitamin E Cancer Prevention Trial (SELECT) Reveals the Need for Better Understanding of Selenium Biology
The Selenium and Vitamin E Cancer Prevention Trial (SELECT) was one of the largest human cancer prevention trials ever undertaken. Designed to examine the role of selenium and vitamin E in preventing prostate cancer (1) as a double-blind study, SELECT administered daily 200 micrograms of selenium in the form of pure l-selenomethionine, 400 IU of synthetic d,l-α-tocopherol (vitamin E), a combination of these two components, or a placebo to four approximately equally divided groups. SELECT enrollment was undertaken between August 22, 2001 and June 24, 2004, and involved 35,533 healthy males from more than 425 participating locations in the United States, Puerto Rico, and Canada. The baseline ages of males selected were fifty years or older for African Americans, and fifty-five years or older for all others. No significant differences in prostate cancer incidence were observed in any of the groups; however, slight but statistically non-significant increases were observed in prostate cancer risk within the vitamin E group and in type 2 diabetes mellitus within the selenium group. Therefore, although SELECT was planned to include a twelve year intervention period, intervention was discontinued after a median period of 5.46 years (2).
SELECT clearly establishes that selenium in the form of l-selenomethionine, when administered to the diet of healthy American males fifty years of age and older, does not prevent prostate cancer. This important observation raises questions as to why SELECT’s findings are different from those of earlier trials (3–8) and numerous animal studies (9, 10) that also employed selenium, but showed dramatic reductions in certain forms of cancer. For example, the Nutritional Prevention of Cancer (NPC) trial was undertaken to examine the role of selenium in preventing skin cancer (5–7). Each patient taking part in the study had a prior incidence of skin basal and/or squamous cell carcinoma. Even though the NPC trial did not decrease skin cancer incidence (6), it found dramatic reductions in other forms of cancer, as well as in cancer mortality. NPC initially reported that prostate cancers were reduced 63%, colorectal cancers 58%, and lung cancers 48% (5). A subsequent analysis of the complete NPC trial data reported that selenium supplementation reduced total and prostate cancer incidence but did not reduce lung or colorectal cancer incidence significantly (6). In addition, NPC had two limitations: it involved a relatively small number of subjects (1,312) and was initially planned to examine the role of selenium in skin cancer prevention, thus considering risk factors only for that endpoint in randomizing subjects. In spite of these factors, the NPC trial, along with the other studies noted above, provided the impetus for undertaking the much larger SELECT. Other trials (also noted above) employing vitamin E provided the basis for using this vitamin in SELECT (3, 4, 8). An exciting anticipation for SELECT was the elucidation of many other possible roles of selenium and vitamin E in mitigating other cancer forms and or other diseases such as their roles in preventing cardiovascular disease.
To understand what SELECT tells us about the role of selenium in cancer prevention, one should examine differences and similarities between SELECT and the earlier clinical trials and take into consideration results from many laboratory animal studies, almost all of which have shown protective effects of selenium. Although the SELECT investigators addressed some of these questions and possible reasons for the discrepancies, further analysis is appropriate. We see SELECT as differing from other clinical trials of selenium in two ways: it used a different form of selenium, and it used subjects of higher selenium status. Because of our expertise in selenium rather than in vitamin E, this commentary focuses mainly on the role of selenium in cancer.
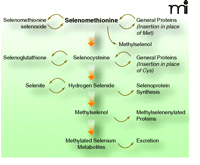
SELECT used pure l-selenomethionine as the intervention agent (1, 2), whereas other human trials and animal studies have demonstrated anti-tumorigenic efficacy for selenite and selenium-enriched baker’s yeast. Although l-selenomethionine represents the major form of selenium in high-selenium yeast, that product has been shown to include other chemical forms of selenium (11). Selenomethionine provides selenium for selenoprotein biosynthesis via the transsulfuration pathway, but it can be diverted from that pathway into general protein synthesis—in lieu of its sulfur-analog, methionine—and it can be used in a number of other ways (Figure 1⇓). This differentiates selenomethionine from other selenium compounds, which cannot be so diverted and, therefore, are methylated to form methylselenol, a presumptive antitumorigenic metabolite methylselenol (12).
The subjects enrolled in SELECT had higher initial plasma levels of selenium than those in the NPC trial (135 ng/ml and 113 ng/ml, respectively). The subjects in the NPC trial were selected, in part, on the basis of their having relatively low serum selenium levels (5); it was in this cohort that selenium supplementation was effective in reducing cancer risks. Moreover, recent analyses show that the treatment effect in the NPC trial was restricted to those with lower baseline plasma selenium concentrations (6, 7). It is of interest to note that the serum selenium levels of individuals in most European countries, even those supplemented with selenium, are lower than in unsupplemented Americans (13). If selenium supplementation can reduce cancer risk in low-selenium subjects, then Europe would have been a better place to conduct a cancer prevention trial at the SELECT scale.
The termination of the active intervention phase of SELECT was widely featured in on-line and printed media, including well-regarded newspapers such as The New York Times, The Washington Post, and USA Today. The American Association of Retired Persons published a short report titled “Selenium, Vitamin E Don’t Help After All.” We consider the widespread use of blanket statements regarding the null SELECT results to miss both the discrepancy noted above and the question raised by that discrepancy: that is, who can benefit from selenium supplementation? However, SELECT, when compared to other findings showing selenium as a cancer chemopreventive agent, has taught us a great deal, and its results should both modify our understanding of the role of this element in cancer and suggest better strategies for targeting selenium to the human population.
For the most part, human clinical trials have been undertaken with little understanding about how selenium acts at the molecular level and the overall potential negative consequences of administering this nutritional micronutrient to such a large number of individuals. Recent studies have shown that some selenium-containing proteins (selenoproteins), such as thioredoxin reductase 1 (TR1), may have roles in preventing cancer, but once the malignancy is initiated, TR1 may actually contribute to progression of the disease [see (14) and references therein]. Other studies in mice have shown that selenium deficiency may inhibit some forms of cancer (15, 16). Numerous studies involving human selenoproteins have shown a direct correlation between one of the polymorphic forms and cancer risk (17–20). These studies suggest the need to learn more about the biology of selenium before administering this element to humans in such large numbers involving clinical trials (Figure 2⇓). Furthermore, another factor to consider is the costs involved that are only a fraction in animal studies of what they are in human clinical trials.
Clearly, our understanding of selenium biology is, at the very least, incomplete. Only recently have we learned the mechanism of selenocysteine biosynthesis in humans (21) and identified the human set of selenoprotein genes (22). Functions of at least half of these gene products are not known and the mechanism of the differential regulation of various selenoproteins with varying selenium in the diet is still unresolved. Although much progress has been made recently in understanding the role of polymorphic sites in selenoprotein genes, further studies are needed. A better understanding of selenium biology requires additional mechanistic, genetic and other experimental approaches, which should be informative in designing clinical studies for elucidating specific roles of selenium in human biology. Then, supplementation with selenium could be targeted to a subset of the human population, or even to individuals of a certain genotype, disease state or selenium status, that can benefit most from this micronutrient.
Selenomethionine metabolism in mammals. Major metabolites of selenomethionine are shown. The incorporation of selenocysteine into protein by replacing cysteine is proposed by analogy to other organisms but has not been verified in humans. For further reading on selenomethionine metabolism see (23, 24).
One outcome of SELECT is the need for basic studies to understand selenium biology. Three basic approaches are illustrated in the figure by a nucleotide sequence highlighting the need for additional human genetics and polymorphism analyses, a mouse for the use of animal models and a protein structure for the functional analyses of selenoproteins.
Acknowledgments
The authors express their appreciation to Drs. Gerald Combs, John Milner, Phil Taylor and Cindy Davis for their helpful suggestions. The research in the laboratory of DLH is supported by the National Institutes of Health, NCI Intramural Research Program and the Center for Cancer Research and in the laboratory of VNG by grants from the National Institutes of Health.
- © American Society for Pharmacology and Experimental Theraputics 2009
References

Vadim N. Gladyshev, PhD, is a Charles Bessey Professor of Biochemistry and Director, Redox Biology Center, University of Nebraska, Lincoln. He received his PhD from Moscow State University and did postdoctoral work at the NIH before joining the faculty at University of Nebraska – Lincoln in 1998. His laboratory works at the interface of selenium and redox biology. E-mail vgladyshev1{at}unl.edu
Dolph L. Hatfield, PhD, is Chief of the Molecular Biology of Selenium Section, Laboratory of Cancer Prevention, Center for Cancer Research, National Cancer Institute, National Institutes of Health, in Bethesda, MD. He received his PhD from the University of Texas and did postdoctoral work at the NIH in Marshall Nirenberg’s laboratory and at the Pasteur Institute in Jacques Monod’s laboratory before coming to the NCI where he has been his entire career. E-mail hatfield{at}mail.nih.gov; fax 301-435-4957.