
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
In Search of Analgesia: Emerging Poles of GPCRs in Pain
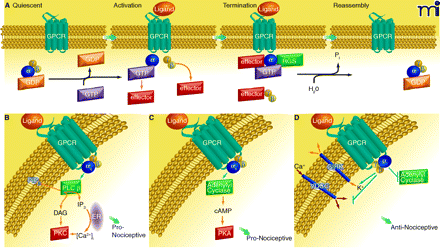
Overview of GPCR signaling. A) Diagram of the cycle of G protein activation and inactivation. In the absence of GPCR signaling (Quiescent), G proteins are present as inactive αβγ trimers; the α subunit is bound to GDP. Binding of ligand to GPCR (Activation) causes a conformational change that promotes binding of the receptor to its preferred trimeric G protein and concomitant displacement of bound GDP by incoming GTP at α subunit. Upon GTP binding, the αβγ trimer dissociates into GTP-bound monomer and βγ dimer, each of which can then interact with respective effectors. Signaling is terminated by the GTPase activity of the α subunit; this GTPase activity can be enhanced by RGS proteins. The α subunit–catalyzed hydrolysis of GTP causes subunits to reassemble into the trimeric G protein. The GPCR is generally desensitized and internalized for recycling or destruction (see text). B–D) Diagrams of the canonical signaling pathways for the major G protein families, as described in the text. (ER, endoplasmic reticulum.)


