 |
 |

Use of the Intrauterine Device by Inner-city Women
Seshu P. Sarma, MD;
Karen Garafalo, PA-C;
William L. Graves, PhD
Arch Fam Med. 1998;7:130-133.
ABSTRACT
Objective To examine the use, effectiveness, acceptability, and continuation rates of the copper T 380 A intrauterine device (IUD) among women attending an inner-city family planning clinic.
Design A 1-year prospective cohort study.
Setting The family planning clinic of Grady Memorial Hospital, which serves the inner-city indigent population of Fulton and Dekalb counties in metropolitan Atlanta, Ga.
Participants A total of 115 women with 1 or more live births who were in a mutually monogamous relationship for the previous year had the copper T 380 A IUD inserted between December 20, 1990, and June 28, 1991.
Main Outcome Measures Follow-up, consisting of history and a pelvic examination, was done 2, 6, and 12 months after IUD insertion to determine the status of the IUD and to detect any complications related to its use. At the end of the study, the continuation rates, reasons for removal, and complications related to IUD use were assessed.
Results Thirty-seven women were unavailable for follow-up. Eight of those were seen in other clinics at Grady Memorial Hospital, but no mention was made of their IUD status. Fifty-six of the remaining 78 women were known to continue IUD use without any complications at the end of the 12 months. Among the 22 women known to have the IUD removed, 8 women cited no problems. Pelvic pain, menometrorrhagia, desire for permanent sterilization, and expulsion of the device were mentioned as reasons for other IUD removals. Pelvic inflammatory disease was not documented in any of the patients during the year of follow-up.
Conclusion With proper screening and counseling, the IUD may be used successfully in selected patients in inner-city family planning clinics.
INTRODUCTION
THE INTRAUTERINE contraceptive device (IUD), despite its many advantages, is used to a limited degree in the United States. This limited use is attributed to the Dalkon Shield experience, which led to the virtual disappearance of all IUD use in the United States in 1986. Researchers had linked the Dalkon Shield, with its filamentous tail, to a substantial increase in the risk of pelvic inflammatory disease (PID).1 However, this is in clear contrast to the widespread use of this contraceptive method in other developed as well as developing countries. In the developing countries, copper-bearing IUDs have been inserted in more than 70 million women in recent years.2-3 In Scandinavia, according to recent epidemiological studies, the IUD was used by 6% to 10% of women aged 20 to 24 years, 12% to 29% of women aged 25 to 29 years, and 30% to 40% of women aged 30 to 44 years.4-5 The widespread use of IUDs in these countries is because of the effectiveness, ease of use, low cost, and safety of these devices. About 80% of women who have an IUD inserted continue to use the device through the first year.6 The first-year continuation rates for subdermal implants are comparable with those for IUDs7 and are much lower than those for oral and injectable contraceptives.8 Despite the concerns raised by the Dalkon Shield litigation, the Food and Drug Administration, even to this day, considers many of the older IUDs safe. The copper T 380 A (ParaGard, Ortho Pharmaceutical Corporation, Raritan, NJ), approved for marketing by the Food and Drug Administration in the United States in 1988, was designed to be more effective than the older-generation copper IUDs. Although recent epidemiological studies9 dispelled concerns regarding PID as a complication related to IUD use, many clinicians have not inserted an IUD since marketing of the Lippes Loop, Tatum T, and Copper 7 was discontinued several years ago.10 In metropolitan Atlanta, most inner-city and publicly funded suburban family planning clinics insert few, if any, IUDs. Many women in these clinics cannot or may not want to use other methods of contraception, such as oral contraceptives and injectables. However, these women are often assumed to be at high risk for acquiring sexually transmitted diseases and are thus deemed unsuitable for IUD use. The availability and actual use of the IUD in inner-city family planning clinics in the United States is not known. Review of the literature fails to show any studies regarding the use of the IUD in this group of patients.
This study examines our experience with the modern copper IUD in women attending a publicly funded, inner-city family planning clinic. The purpose of the study is to evaluate whether the IUD can be used successfully among inner-city clinic populations.
MATERIALS AND METHODS
In the family planning clinic of Grady Memorial Hospital in Atlanta, Ga, 115 women had the copper T 380 A inserted between December 20, 1990, and June 28, 1991. Eligibility for IUD use was based on information obtained at the time of initial history and physical examination (Table 1). Computer records were used to screen for the presence of any sexually transmitted diseases in the preceding years. For patients eligible for Medicaid, the cost of the IUD, its insertion, and follow-up was reimbursed by Medicaid. Some patients received Title X grant support and the cost for a few insertions and follow-up was absorbed by the Fulton-Dekalb County Hospital Authority.
|
|

|
|
Criteria Used in Selecting Patients for Copper T 380 A Insertion*
|
|
|
Extensive counseling, in person by the clinician inserting the IUD and through educational materials, was given to patients enrolled in the study. The importance of mutual monogamy to prevent sexually transmitted diseases and PID was emphasized. Women were advised to have the IUD removed or to use condoms consistently when mutual monogamy was no longer ensured. A thorough physical examination, routine screening for chlamydial and gonococcal infections, a Papanicolau smear, a hematocrit, and a wet smear for the presence of vaginitis were done before inserting the IUD. Most of the IUD insertions were done by the first and second authors (S.P.S. and K.G.), and some were done by the second-year obstetrics and gynecology residents of Emory University School of Medicine, Atlanta, who were rotating through the Family Planning Program. Patients were advised to take 500 mg of naproxyn sodium twice daily to alleviate the dysmenorrhea and menorrhagia often seen in the initial months of IUD use.
Patients were followed up 2, 6, and 12 months after IUD insertion. A pelvic examination to confirm the presence of the IUD and to detect any complications was performed on all patients. Appropriate testing, such as gonorrhea and chlamydia screening, hematocrit, wet smear, and pregnancy testing, were done when necessary. Laboratory results from previous visits since insertion of the IUD were reviewed, and proper treatment was given. Specific criteria were used for removal of the IUD: (1) the patient's request for removal, (2) menometrorrhagia, (3) dysmenorrhea that did not improve with naproxyn treatment, (4) dyspareunia, (5) pregnancy, and (6) PID. At study initiation, a gynecologic infectious disease specialist was consulted, and criteria were established for the diagnosis of PID based on a modified version of the Centers for Disease Control and Prevention's "Criteria for Clinical Diagnosis of PID."11 All 4 of the following criteria should be met: pelvic or abdominal pain, abdominal tenderness with or without rebound tenderness, cervical motion tenderness (uterine and adnexal tenderness), and more than 15 white blood cells per high-power field on wet smear (saline preparation). All 3 of the minimum criteria required in the Centers for Disease Control and Prevention's guidelines for clinical diagnosis of PID, were used in our study. However, since a dislodged or displaced IUD may cause pelvic pain and uterine or cervical tenderness, presence of inflammatory cells on wet smear was added as an additional criterion to make a diagnosis of PID in our study patients. In all laparoscopically verified cases of salpingitis in the Swedish studies,12-13 microscopic examination of a wet smear of the vaginal contents revealed a marked increase in the number of inflammatory cells; ie, inflammatory cells outnumbered all other cellular elements in the smear. In general, the presence of more than 10 leukocytes per high-power microscopic field suggests the presence of an inflammatory response. However, because IUD use causes sterile inflammation in the uterine cavity, more than 10 white blood cells per high-power field on wet mount may not be abnormal in IUD users. Hence, we used presence of more than 15 white blood cells per high-power field on wet smear as an additional criterion for diagnosing PID. Patients who had vaginitis without evidence of upper genital tract infection were treated with appropriate antibiotics without removing the IUD.
Data were collected from the medical charts, computer files, and telephone interviews;  2 and Fisher exact tests were used for statistical analysis. 2 and Fisher exact tests were used for statistical analysis.
RESULTS
One hundred fifteen women had the copper T 380 A inserted in the 6 months between December 20, 1990, and June 28, 1991. African American women composed 80% and Hispanic women accounted for 10.4% of the women receiving IUDs. Seventy-three (63.5%) of the 115 women were younger than 25 years, and 13 women (11.3%) were younger than 20 years. The 2 youngest patients to receive IUDs were 17 years of age, and the oldest patient was 41 years old. Thirty-four (29.6%) of the 115 women were married at the time of IUD insertion.
Regarding the number of sexual partners in the previous 3 years, 81 women (70.4%) had only 1 partner, 33 (28.7%) had 2 to 5 partners, and 1 had more than 5 partners. Eighty-six percent of the women were certain that they were in mutually monogamous relationships at the time of IUD insertion, and 13.9% said that they were monogamous but not absolutely certain whether their partners were having any other sexual encounters.
Twenty-nine women (25.2%) had 1 living child, and 84 women (73.0%) had 2 to 5 children at the time of IUD insertion. Thirty-four women (29.6%) had been taking oral contraceptives, 44 (38.3%) were using barrier methods, and 24 (20.9%) were abstaining at the time of IUD insertion. Thirty women (26.1%) had a history of IUD use. One woman had a history of gonorrhea and another woman had a history of chlamydia in the 3 years before IUD insertion.
Thirty-seven women were unavailable for 1-year follow-up. Eight of these women were seen at Grady Memorial Hospital several times, but no mention of their IUD was made. Seven women still had the IUD in place at 6 months, 5 at 4 months, and 6 at 2 months, but they were unavailable for follow-up afterward. Eleven patients were not seen after insertion of the IUD.
Fifty-six women were known to have the IUD in place at the end of the first year, and 22 women (19.0%) had the IUD removed during the same period. Eight women who had the IUD removed cited no problems with the IUD but did not wish to continue using the device; 6 women mentioned pelvic pain as the reason for removal of the device; 2 women had tubal ligation, hence no need for the contraceptive device; 1 woman had the IUD removed because of menometrorrhagia and another because of dyspareunia. There was 1 case of partial expulsion, and another IUD was accidentally pulled out. One woman presented to the emergency department with a fever, and the IUD was removed. She was later diagnosed as having pyelonephritis. One woman requested removal when she found out that her partner was not monogamous. There were no pregnancies and no documented PID.
For the 78 patients followed up for 1 year, there was no correlation between age, marital status, and number of sexual partners in the 3 years preceding IUD use and the continuation rates of the device beyond 1 year. Seventeen of 63 women 25 years old and younger vs 5 of 52 women older than 25 years had the IUD removed (P=.07); 4 of 34 married women vs 18 of 81 unmarried women had the IUD removed (P=.34); 14 of 81 women who had 1 sex partner in the 3 years preceding IUD insertion vs 8 of 34 women with more than 1 sex partner in the same period had the IUD removed (P=.81).
Pelvic pain as a reason for removal of the IUD was not seen any more frequently in women 25 years of age and younger (4 of 17 women) than in women older than 25 years (2 of 5 women) (P=.58, Fisher exact test). One of 4 married women vs 5 of 18 unmarried women had the IUD taken out because of pelvic pain (P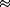 1.00, Fisher exact test). Four of 14 women who had 1 sex partner in the 3 years preceding IUD insertion vs 2 of 8 women with more than 1 sex partner in the same period had the IUD removed because of pelvic pain (P 1.00, Fisher exact test). Four of 14 women who had 1 sex partner in the 3 years preceding IUD insertion vs 2 of 8 women with more than 1 sex partner in the same period had the IUD removed because of pelvic pain (P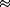 1.00, Fisher exact test). 1.00, Fisher exact test).
The diagnosis of PID was entertained, but not confirmed, in 3 women who had pelvic pain: 1 at 10 weeks, another at 16 weeks, and the third at 6 months. However, in all 3 women, the gonorrhea and chlamydia test results were negative, and a wet smear failed to reveal a significant number of white blood cells (>15 per high-power field). None of the women had any abdominal tenderness, and only 1 woman had mild cervical and uterine but no adnexal tenderness. The IUD was removed and treatment with doxycycline and metronidazole was instituted in all 3 patients by clinicians not involved in this study. None of these 3 patients met the criteria established at the beginning of our study for the diagnosis of PID. Follow-up data for these patients are not available.
One woman who had a history of deep venous thrombosis before insertion of the IUD died of massive pulmonary embolism 18 months after IUD insertion. At the time of autopsy, no problems related to IUD use were noted. Another woman developed abdominal pain, nausea, and vomiting 5 days after IUD insertion. She was given trimethoprim-sulfamethoxazole because she was suspected of having a urinary tract infection, and the IUD was removed even though there was no evidence of pelvic infection. Later she was diagnosed as having pyelonephritis when her urine and blood cultures yielded Proteus mirabilis. She was hospitalized and treated with intravenous ampicillin successfully.
Among the 13 teenagers in the study, 9 were still using the device without any problems at the end of the study. There was 1 case of partial expulsion, and 1 patient had the IUD removed without giving the reason. Two 19-year-old patients were using the device without any problems 8 and 9 months after IUD insertion, but they were unavailable for follow-up at the end of the study.
The 2 women who had a history of gonococcal and chlamydial infection in the 3 years before IUD insertion continued IUD use until the end of the study.
COMMENT
The use of the IUD as a contraceptive method declined precipitously in the United States in the 1980s. In a study of physicians in San Diego, Calif, the most common reason given for not inserting IUDs was concern about medicolegal liability.14 However, the device is undergoing a renaissance in the United States in the 1990s because recent epidemiological studies15 have shown no relationship between IUD use and PID except in the immediate postinsertion period. Meta-analysis of data from 12 randomized studies by Farley et al15 revealed that PID among IUD users is most strongly related to the insertion process and to background risk of sexually transmitted disease. It is now believed that if a woman using the device contracts PID beyond the first month of use, she may have developed PID even if she were not using the device.
The introduction of a new copper-containing IUD into the American market in 1988 has generated considerable interest among clinicians providing reproductive health care. Many experts in contraception believe that the current IUDs are so safe and effective that clinicians need not be concerned about medicolegal issues, provided that proper screening and counseling are given to patients requesting the IUD.16-17 Moreover, the fact that the IUD, once inserted, does not require patient compliance and that the copper T 380 A does not need to be replaced for 10 years, makes it an attractive method for many sexually active women of all ages. Another important issue is cost-effectiveness. Through economic modeling and third-party payers' databases, Trussel et al18 concluded that the most cost-effective reversible contraceptive methods are the IUD, the contraceptive implant, and the injectable contraceptive. They noted in their study that despite high initial costs, the IUD and the implants become extremely cost-effective with increased duration of use. Nonetheless, there are many practice settings in which the IUD is considered inappropriate based solely on concerns about certain sexual behaviors of the patient population. Such is the case in several inner-city and many publicly funded family planning clinics.
Our study shows that there are many single women in publicly funded programs for whom the IUD may be an appropriate contraceptive. Many such patients are in mutually monogamous relationships even though they are not married. Although a third of our patients were unavailable for follow-up, analysis of the available data revealed no major complications. We cannot assume that patients who were unavailable for follow-up were without problems. However, since Grady Memorial Hospital is the primary source of health care for the patients it serves, failure of patients to return for follow-up visits probably means that they have not had any problems with the IUD. More research is needed to ascertain that age, marital status, and past sexual history have no bearing on successful use and on the continuation rates of the IUD.
It is imperative that the woman receiving the IUD is in a mutually monogamous relationship during IUD use. Should there be a change in the status of her relationship, she should protect herself against sexually transmitted infections by consistent use of condoms or have the IUD removed. This information should be clearly conveyed to the patients to prevent complications related to sexually transmitted diseases during IUD use. Even in the best setting, only about 80% of the women receiving the IUD continue to use it at the end of 1 year,6 which is higher than the continuation rates for oral and injectable contraceptives. Women in the United States today have a wide array of effective contraceptives to choose from. Yet, those aged 20 to 34 years have the highest abortion rates in the industrialized world.17 High abortion rates and many unwanted pregnancies may be reduced by increasing judiciously the use of the IUD among appropriate candidates. More research and education are needed to encourage the use of the IUD in publicly funded family planning clinics across the United States. We strongly believe that women in these clinics as a group should not be denied the option of using the IUD based solely on the perception that they are considered to be at "high risk" for sexually transmitted diseases. With proper screening and counseling, the IUD may be successfully used in selected patients in publicly funded family planning clinics too.
AUTHOR INFORMATION
Accepted for publication January 13, 1997.
We thank Lee Warner for his help with data management, Ann Strickland and Linda Winders for their help with contacting patients and retrieving data from patients' files, and Herbert B. Peterson, MD, chief of the Women's Health and Fertility Branch at the Centers for Disease Control and Prevention, Atlanta, Ga, for his invaluable advice and support.
Reprints: Seshu Sarma, MD, PO Box 26134, Morehouse School of Medicine, Grady Health System, 80 Butler St, Atlanta, GA 30303.
From the Women's Health Care/Family Planning Clinic, Grady Health System (Dr Sarma), and the Department of Gynecology/Obstetrics, Emory University School of Medicine (Ms Garafalo and Dr Graves), Atlanta, Ga. Dr Sarma is now with the Department of Obstetrics and Gynecology, Morehouse School of Medicine, Atlanta.
REFERENCES
1. Burkman RT. Association between intrauterine device and pelvic inflammatory disease. Obstet Gynecol. 1981;57:269-276.
WEB OF SCIENCE
| PUBMED
2. Sivin I, Greenslade F, Schmidt F, Waldman SN. The Copper T 380 A Intrauterine Device: A Summary of Scientific Data. New York, NY: Population Council; 1993.
3. Bergsjo P. Update on the intrauterine contraceptive device. Acta Obstet Gynecol Scand. 1992;71:163-165.
WEB OF SCIENCE
| PUBMED
4. Riphagen FE, von Schoultz B. Contraception in Sweden. Contraception. 1989;39:633-642.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
5. Skjeldestad FE. Choice of contraceptive modality by women in Norway. Acta Obstet Gynecol Scand. 1993;72:48-52.
6. Sivin I, Tatum HJ. Four years of experience with the T Cu 380A intrauterine contraceptive device. Fertil Steril. 1981;36:159-163.
WEB OF SCIENCE
| PUBMED
7. McCauley AP, Geller JS. Decisions for Norplant program. Popul Rep. 1992;4 (ser K).
8. Schwallie PC, Assenzo JR. Contraceptive use-efficacy study utilizing medroxy-progesterone acetate administered as an intramuscular injection once every 90 days. Fertil Steril. 1973;24:331-339.
PUBMED
9. Lee NC, Rubin GL, Borucki R. The intrauterine device and pelvic inflammatory disease revisited: new results from the Women's Health Study. Obstet Gynecol. 1988;72:1.
WEB OF SCIENCE
| PUBMED
10. Grimes DA. IUD insertion: a clinical refresher. Female Patient. 1989:14:51-54.
11. CDC. Sexually Transmitted Diseases Clinical Practice Guidelines. Atlanta, Ga: Centers for Disease Control; 1989.
12. Westrom L. Diagnosis, Aetiology, and Prognosis of Acute Salpingitis [thesis]. Lund, Sweden: Studentlitteratur; 1976.
13. Svensson L, Westrom L, Ripa KT, Mardh PA. Differences in some clinical and laboratory parameters in acute salpingitis related to culture and serologic findings. Am J Obstet Gynecol. 1980;138:1017-1021.
WEB OF SCIENCE
| PUBMED
14. Kooiker CH, Scutchfield FD. Barriers to prescribing the Copper T 380 A intrauterine device by physicians. West J Med. 1990;153:279.
WEB OF SCIENCE
| PUBMED
15. Farley TMM, Rosenberg RJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1991:339:785-788.
16. Chez RA, Grimes DA, Tatum H. IUDs: under-used and misunderstood. Contemp Obstet/Gynecol. 1991;36:73-92.
17. Pasquale SA. Helping patients make informed contraceptive decisions. Contemp Obstet/Gynecol. 1994;39:9-25.
18. Trussel J, Leveque JA, Koenig JD, et al. The economic value of contraception: a comparison of 15 methods. Am J Public Health. 1995;85:494-503.
WEB OF SCIENCE
| PUBMED
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
Relationship Between Use of the Intrauterine Device and Pelvic Inflammatory Disease
Sarma
Arch Fam Med 1999;8:197-197.
FULL TEXT
|