
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Splice Variants of GPCRs
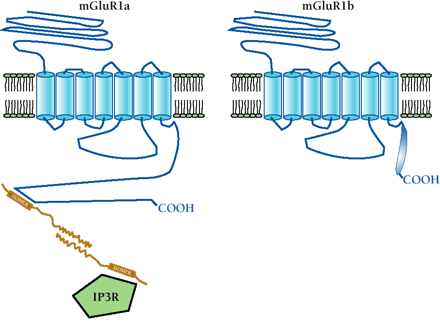
Potential role of protein–protein interactions in the differentiation of splice variant function. C-terminal splice variation may result in distinct protein-binding motifs, leading to specific interactions with intracellular proteins. This mode of functional regulation, mediated by splicing, is shown in the schematic for two variants of mGluR1. Specifically, the immediate-early gene product Homer is schematized to interact with mGluR1a but not the shorter C-terminal splice variant mGluR1b. Homer proteins bind to a proline-rich consensus motif in the C-terminal tail of mGluR1a that is spliced out of mGluR1b. The homodimerization of Homer through its coiled-coil domains is proposed to physically link the mGluR1a receptor to the inositol 1,4,5-trisphosphate receptor (IP3R), which also contains a Homer binding motif. Because the mGluR1b (shown), mGluR1c, and mGluR1d C-terminal splice variants do not contain a Homer binding motif, they would not be expected to associate with Homer proteins. Whether these C-terminal splice variants specifically interact with other proteins is not yet known. Other protein-binding sequences that may be introduced into or excluded from GPCRs are discussed in the text.
This Article
-
MI June 2001 vol. 1 no. 2 108-116


