Spindle Poisons and Cell Fate: A Tale of Two Pathways
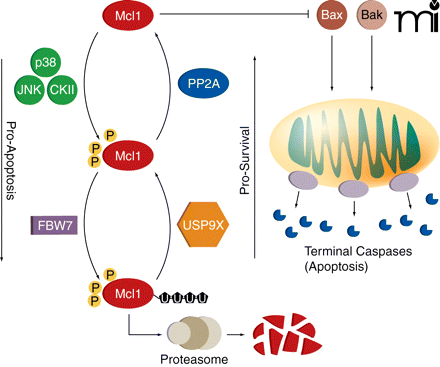
During prolonged mitosis, Mcl1 prevents the induction of apoptosis by inhibiting the binding of Bak and Bax to the mitochondrial membrane. However, Mcl1 levels fall over time. The protein kinases p38, Jun N-terminal kinase (JNK), and casein kinase II (CKII) phosphorylate Mcl1, driving its interaction with FBW7, the substrate binding component of a ubiquitin ligase complex. Ubiquitinated Mcl1 is then degraded by the proteasome. The deubiquitinase USP9X and protein phosphatase PP2A can promote Mcl1 stability by removing ubiquitin side-chains and dephosphorylating Mcl1, respectively. When Mcl1 concentrations fall low enough, Bak and Bax form pores in the mitochondrial membrane, resulting in the release of cytochrome c and terminal caspase activation and in apoptosis.



