Life Cycle of A Block Buster Drug
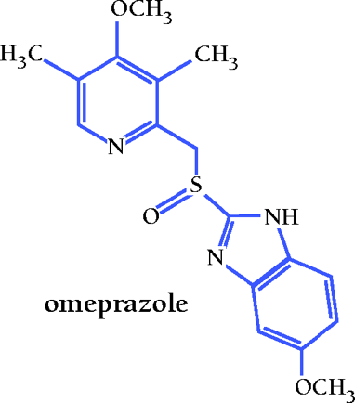
Discovery and Development of Omeprazole (PrilosecTM)
- Barry A. Berkowitz, PhD and
- George Sachs, MB, ChB, DSc
Abstract
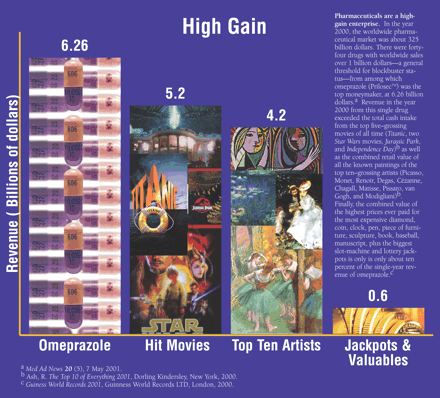
Blockbuster drugs share a variety of common features, among which is the tendency to create entirely new markets. For example, an early “informed” estimate of the potential market size for the hypothetically “perfect” peptic ulcer drug was thirty-five million dollars. Based on current sales, however, we reckon this hypothesis to have underestimated the actual market demand for omeprazole (PrilosecTM) by about 400-fold. Similarly, prior to the introduction of the “retired” blockbusters chlordiazepoxide and diazepam (LibriumTM and ValiumTM), the market for “minor tranquilizers” in the treatment of anxiety and neurosis did not exist. Thus, once an emerging blockbuster seems to be therapeutically working, it is not unusual for diagnostic rates of the disease for which it is indicated and efficacious to actually increase. Top blockbuster drugs generally have or appear to have a high margin of safety.
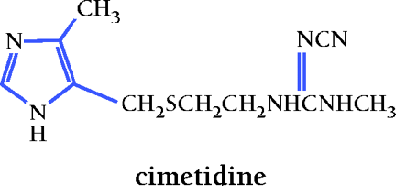
Gastric Acid Secretion and the Advent of Cimetidine
Although it had been known for almost 200 years that gastric acid secretion was regulated, and for about 50 years that histamine was one mediator of such regulation (1), the pharmacological manipulation of gastric secretion was not achieved until 1972. Black et al. (2) utilized the concept of selective histamine receptor subtypes to discover the H2-receptor antagonist cimetidine (TagametTM). This discovery established new vistas for the effective treatment of gastric and duodenal ulcers, heartburn, and gastritis, and launched the market for acid-controlling substances and gastrointestinal drugs that today exceeds twelve billion dollars per year (3).
Nevertheless, cimetidine left room for improvement. The drug necessitated multiple dosing and was associated with undesirable fluctuations in gastric acid levels. In addition, it failed to adequately treat gastro-esophageal reflux disease (GERD) as well as the excessive acid secretion that occurs in pathological hypersecretory conditions (e.g., Zollinger-Ellison syndrome), so that a medical need and a potentially large and growing market went unattended. In fact, as duodenal ulcer disease declined in response to cimetidine, GERD grew in relative significance and increased market demands for acid-related therapy that had been unpredicted in market surveys. There was thus a need for a new drug.
The Development of Omeprazole
Systematic drug discovery requires a biological hypothesis. In 1968, George Sachs and his collaborators at SmithKline & French began work that established an H+,K+-ATPase as the proton pump that moves acid across the gastric mucosa and gastric parietal cells (4–7). Furthermore, Sachs hypothesized that the H+,K+-ATPase proton pump might be a key drug target for control of gastric secretion of acid.
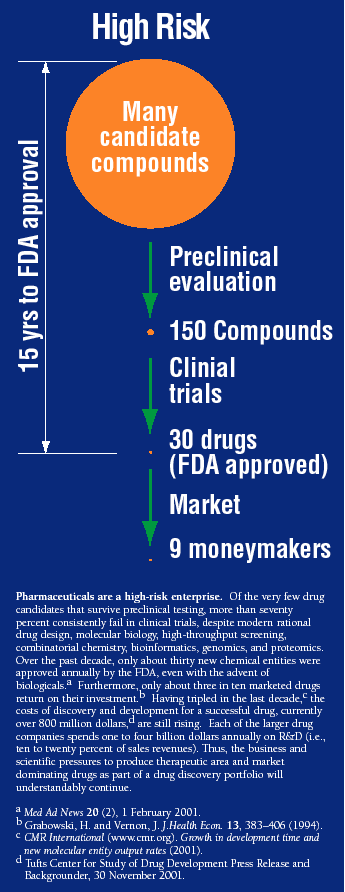
In addition to a biological hypothesis (e.g., that the H+, K+ ATPase is a suitable target for the intervention of gastric acid secretion), systematic drug discovery also requires a chemical hypothesis. It is not surprising that, prior to omeprazole, no drug had established effective, long-term and long-lasting control of gastric acid secretion. Among the formidable issues that plagued the pharmacological manipulation of gastric secretion was the acidic environment of the stomach and parietal cells, along with the concomitant needs for target selectivity, drug stability, and effective drug delivery. In addition, a significant body of intellectual property around H2-receptor antagonists had been emerging. In industry, as in academia, new ideas, no matter how innovative, must compete for resources against many ongoing programs, and without effective internal champions, even the most excellent of ideas, many of which will later prove fruitful in the hands of others, may not be funded. Thus, a program of rational drug design, along with the commitment to understand the physiological mechanisms of acid secretion in the stomach, was crucial to the discovery and development of omeprazole as a blockbuster.
At Astra Pharmaceuticals (formerly know as AB Hässle), in Sweden, the search for drugs that might improve upon the emerging H2-receptor blockers for control of acid secretion began in the mid 1970s. Because the work of Jim Black and colleagues at SmithKline had suggested that a substituted imidazole (e.g., cimetidine) was important for acid control by H2-receptors, the Astra group added a benzimidazole moiety to pyridine-2-thioacetamide, a known anti-secretory agent. Chemically, the resulting derivative and its congeners, including omeprazole, were sufficiently novel so that an argument could be made in favor of their patentability. In addition, it was eventually realized that the weakly basic nature of the benzimidazoles might contribute to drug effectiveness by permitting their accumulation in acidic environments (e.g., at the canalicular spaces of gastric parietal cells, having a pH of about 1), precisely where acid control was needed. Indeed, substituted benzimidazoles with pKa values around 4.0 accumulate about 1000-fold in these low-pH spaces and thus result in great organ selectivity. Moreover, benzimidazoles behave as prodrugs, undergoing an acid-catalyzed rearrangement that provides a reactive sulfenamide species that inhibits the H+,K+-ATPase in the gastric milieu (8). Finally, these agents covalently bind to their target, so that their inhibitory activity outlives the presence of prodrug in the blood (see mechanism) (1,9). This efficacious mechanism of action was unknown prior to the identification of the benizimidazoles as selective proton pump inhibitors.
The Lesson of Collaboration
Whereas collaboration between groups of investigators is often welcomed in academia, it is required in industry. At the same time, industry is increasingly focusing on the application of technologies and marketing. Generally—and there are some exceptions—industry cannot undertake the breadth and depth of basic research that characterizes academia. Another difference between academic and industrial science is that in academia, it is acceptable—indeed desirable—to engage in experiments that result in both positive and negative data in order to address hypotheses in an unbiased manner. In industry, the search for scientific truths is also desirable and necessary, but of itself, good science is not always a sufficient prescription to ensure enterprise or business success. Thus, interactions between academia and industry can provide powerful complementarities and synergies for drug discovery and development; such was the case for omeprazole.
While Sachs (10) was unraveling the molecular mechanisms that underlie the regulation of acid secretion, increasingly focusing on proton pumps and H+,K+-ATPase, Astra scientists, including Sjostrand, Brandstrom, Lindberg, and Fellenius, were seeking new chemical compounds to suppress acid production (11). From discussions at a scientific meeting in Sweden in 1977, Sachs and Astra scientists began the collaboration that eventually yielded omeprazole. Specifically, early success in the suppression of acid secretion by the substituted benzimidazoles (e.g., timoprazole and picoprazole) synthesized by Astra scientists was mechanistically complemented by Sachs's work on the gastric H+,K+-ATPase. And although initial drug leads proved to be unstable, too toxic, or nonselective, risk–return considerations propelled the project, and omeprazole was discovered in 1978. The compound began to be tested in humans in 1983 and 1984 (4, 13) and was approved in the USA for marketing in 1989. Twelve years had thus passed between the beginning collaboration and regulatory approval.
Collateral Gain: Nothing Succeeds like Success
Successful drugs often stimulate additional research. The therapeutic and marketing success of cimetidine, along with the tidal wave of growing basic knowledge of acid regulation, certainly catalyzed the success of omeprazole and other proton pump inhibitors. Correspondingly, there has been a dramatic increase in the use of proton pump inhibitors to define important biological processes and better treat pathophysiology. Subsequent to the discovery and success of omeprazole, there have been dramatic gains in our understanding, not only of acid regulation, but also of membrane transport, ATPases, and pump mechanisms that inform our use of established drugs, and may guide us in defining potentially important therapeutic targets. Digitalis, for example, a milestone drug in the history of pharmacology, targets a sodium pump. Yeast proton pumps, moreover, are receiving attention in the development of antifungal agents, and bacterial bioenergetics may similarly prove susceptible to antibiotics that interfere with ion transport. Thus, it is not entirely surprising that ion and membrane transport processes are now among the hot areas of functional genomics, proteomics, and drug discovery.
The combination of proton pump inhibitors with selected antimicrobials has served as the cornerstone for the successful treatment of H. pylori infection. Important medical advances have thus been made not only in the therapy of heartburn, gastritis, and gastric and duodenal ulcers, but also in preventing the progression of many such cases into gastric cancer. Success in gastrointestinal therapeutics has also strengthened the case for investigating the roles of H. pylori and other microorganisms in important chronic diseases that have not traditionally been attributed to infectious agents.
The cost effectiveness of proton pump inhibitors is a strong example of how pharmaceutical development can positively affect society at large in addition to patients per se. Proton pump inhibitors, alone or in combination therapy, have had a very positive pharmaco-economic effect in interrupting the trajectory of escalating costs associated with gastritis, ulcers, and adenocarcinoma. The estimated annual costs to American society for gastro-esophageal reflux disease are over ten billion dollars, which includes medication expense, time lost from work, doctors' fees, and hospital visits (14, 15). Moreover, it has been estimated that the use of proton pump inhibitors has substantially lowered in-patient costs; compared to surgery, for example, drug therapy is estimated to reduce costs for patients with erosive reflux esophagitis by 50% (16).
The Limits of Blockbuster Success
Even blockbuster discovery and development, the tradition upon which the pharmaceutical industry was founded, cannot guarantee long-term business success and perpetual corporate independence. Since the discovery of the H2-receptor blockers, SmithKline & French has merged with a number of companies, including Beecham, followed by GlaxoWellcome, their long-time rival in the area of acid-related diseases; Astra has now merged with Zeneca. Blockbusters and significant business growth create demands and pressures for even greater growth, often met in today's environment by a full complement of scientific and business “weapons,” including not only drug discovery, but also alliances, acquisitions, and mergers.
In 2001, the patent for Prilosec expired, and generic omperazole will likely be manufactured and sold by additional companies. This transition generally sounds the death knell of a blockbuster. Nevertheless, Prilosec, like all blockbuster drugs, leaves a proud history of accomplishment as a drug, as a productive biological tool, as a driver of technology, and as an enterprise, and continues a legacy as a provider of health and therapy.
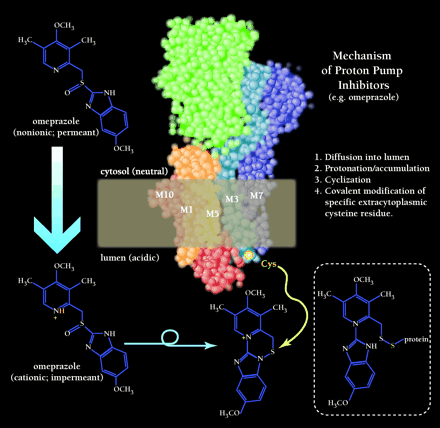
Artist rendering of the 3D model of the catalytic subunit of the H+,K+-ATPase present in the gastric parietal cell canalicular membrane. (Membrane is indicated by brown rectangle; M1, M3, M5, M7, and M10 indicate transmembrane domains). The mechanism of enzyme inhibition, subsequent to absorption of prodrug from blood stream, is indicated in four steps. (Large white/blue arrow indicates movement of prodrug across the membrane into the lumen. Accumulation of omeprazole in the acidic lumen occurs via ionization (i.e., protonation; indicated in yellow), which prevents retrograde diffusion across the membrane. The cationic species cylizes to produce a sulfenamide that undergoes nucleophilic attack (indicated by yellow arrow) by a specific extracytoplasmic cysteine residue. The inactive, covalently modified H+,K+-ATPase is indicated in the lower right corner.
Barry A. Berkowitz, PhD, is Corporate Vice President, Albany Molecular Research, Inc. and Scientist in Residence at Northeastern University. He has held both research and executive positions at the Roche Institute of Molecular Biology and SmithKline, and was Founder and Chief Executive Officer of several biotechnology companies: Magainin (now Genaera); Myco/Chemgenics (acquired by Millennium); Strong Pharmaceuticals (now Fibrogen); and New Chemical Entities (acquired by Albany Molecular Research). He continues to enjoy drug discovery and the development of people, companies, and ideas. E-mail strongpharmaceuticals{at}compuserve.com
Acknowledgments
We thank Prince Bosque-Hamilton for research efforts and Dr. Wallace Dairman for comments on the manuscript.
- © American Society for Pharmacology and Experimental Theraputics 2002
References

George Sachs, MB, ChB, DSc, is Director of the Membrane Biology Laboratory, Center for Ulcer Research, Department of Veterans Affairs, Wardsworth Hospital, Los Angeles, California. He continues to conduct research and consult in the molecular biology of membrane transport, gastrointestinal function and novel drug discovery. E-mail gsachs{at}ucla.edu.