
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Developing a New Generation of TNFα Antagonists for the Treatment of Rheumatoid Arthritis
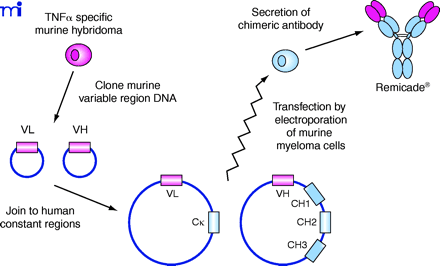
Figure 2.
Derivation of chimeric mouse–human MAbs such as Remicade® using recombinant DNA technology. Heavy and light chain variable cDNAs are cloned from a hybridoma cell clone producing the desired mouse MAb . These variable sequences (VL and VH) are inserted into plasmid vectors containing the desired human light and heavy chain constant domain sequences (the Cκ and G1 CH1, CH2 and CH3, respectively) and transfected into myeloma cells. The antibody secreted by these cells is now chimeric, containing variable protein domain derived from the original mouse antibody fused to human constant protein domains.


