
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Two Birds with One Stone: Novel Glucokinase Activator Stimulates Glucose-Induced Pancreatic Insulin Secretion and Augments Hepatic Glucose Metabolism
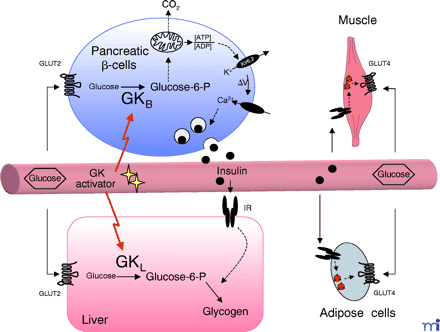
Central role of glucokinase (GK) in whole-body glucose homeostasis. In pancreatic β-cells, B-type GK (GKB) constitutes part of the glucose sensor. Glucose is transported into the cells by the glucose transporter isoform GLUT2, to be phosphorylated by GK yielding glucose-6-phosphate. Glycolysis and oxidative metabolism of glucose increases the ATP:ADP ratio, leading to inactivation of the Kir6.2 potassium channel and to subsequent depolarization of the membrane. Following influx of Ca2+ through a voltage-gated Ca2+ channel, insulin-containing storage vesicles fuse with the plasma membrane, causing the release of the hormone into the blood stream. In the liver, production of glucose-6-phosphate by L-type GK (GKL) precedes storage of glucose as glycogen, which is stimulated by insulin. In adipose and muscle cells, insulin stimulates glucose uptake and metabolism by triggering the translocation of the glucose transporter isoform GLUT4 from intracellular storage vesicles to the plasma membrane. The novel GK activators augment both glucose-induced insulin secretion in β-cells and hepatic glucose metabolism, and as a result lead to improved clearance of glucose from the blood stream.


