
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Ceramide-1-phosphate: The “missing” link in eicosanoid biosynthesis and inflammation
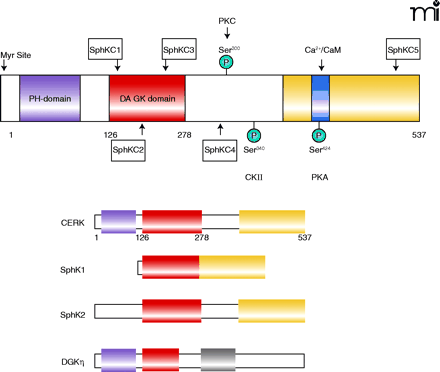
The structure of CERK. The structural domains of CERK include the PH domain (purple), the diacylglycerol kinase homology domain containing subdomains C1 through C3 (red), and the C-terminal domain (yellow) containing the C5 subdomain and the calmodulin interaction site (blue). Conservation of some of these domains is reflected among other kinases, including sphingosine kinase 1 (SphK1), sphingosine kinase 2 (SphK2), and diacylglycerol kinase η (DGKη). Modification sites within the CERK structure include a casein kinase II phosphorylation site (CKII) at Ser340, a cAMP-dependant phosphorylation (PKA) site at Ser424, and a protein kinase C (PKC) phosphorylation site at Ser300.


