
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
CALCIUM: A Role for Neuroprotection and Sustained Adaptation
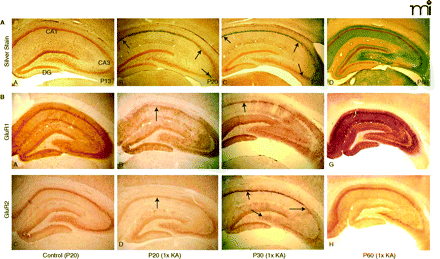
Maturational switch in neuronal vulnerability after status epilepticus. A. Photomicrographs of age-dependent kainic acid (KA) seizure-induced neurodegeneration (silver impregnation 3 days after status epilepticus). (A) KA seizures at postnatal day 13 (P13) resulted in control labeling and minimal or no argyrophilia (silver staining). (B) At P20, 1×KA produced robust injury that was preferential to the CA1 subregion (between arrows); few scattered CA3a neurons were labeled by the silver stain (arrow). (C) At P30, the CA1 was still preferentially marked by the silver stain (arrows), but many scattered neurons of the CA3a were also argyrophilic and the hilus was spared (arrow). (D) At P60, the pattern reversed such that CA3a-c and hilus were predominantly affected with high intensity labeling of dendritic arborizations (grey color); few CA1 neurons were labeled. 40× magnification. B. P20 rats: Control photomicrographs of GluR1A (A) and GluR2B (C) show uniform and intense immunolabeling of the hippocampus at 72 hrs after 1×KA, GluR1B protein is markedly decreased in CA1 and dentate gyrus (DG) but is sustained in the CA3 and ventral DG (B). GluR2B protein is decreased in CA1 (D) but much less so than that of GluR1A (B). P30 rats: GluR1A immunolabeling (E) after 1×KA shows significant depletion of protein in CA1 neurons; GluR1A was sustained in surviving CA3 neurons. In contrast, at P30 GluR2B (F) protein was increased in CA1 and marked the injured neurons. GluR2B protein was decreased in surviving CA3 subregions. (G), In contrast, at P60, GluR1A immunodensity was significantly dense in CA1 and CA3 whereas GluR2B (H) was decreased in the CA3 and hilus. Modified from (32, 45). Reprinted with permission.


