
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Phase 0 Clinical Trials in Cancer Drug Development: From FDA Guidance to Clinical Practice
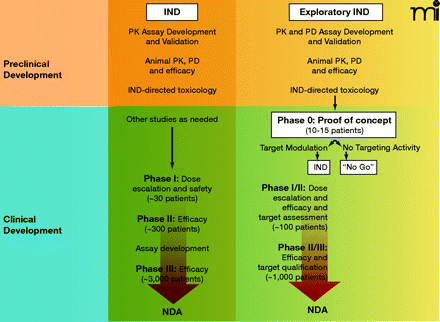
Key components of cancer drug development from preclinical evaluation through NDA submission following traditional (Phase I) or exploratory IND (Phase 0) clinical pathways. The objective of preclinical studies performed to support a traditional IND is to estimate a starting dose for Phase I trials which is both safe and allows for the possibility of clinical benefit. Subsequent Phase II and III trials continue to monitor drug safety and bioavailability as well as evidence of efficacy. Large numbers of patient volunteers are needed at each clinical stage, and the drug can fail for unacceptable toxicity, poor pharmacokinetics, or lack of efficacy at any point during this pathway. The objective of Phase 0 trials is to anticipate clinical failure and potential efficacy. Study endpoints focus on target modulation, necessitating development and validation of plasma drug (pharmacokinetic) and preclinical biomarker (pharmacodynamic) assays during preclinical evaluation. If the drug shows promise, the experimental IND is closed and further development proceeds under a traditional IND to explore drug safety. Phase I/II trials (target assessment studies) of molecularly-targeted agents can then be performed with a qualified pharmacodynamic assay that could enhance the basis on which future development decisions for the agent are made. Progression to Phase II/III randomized trials would then occur with a stronger scientific base. PK, pharmacokinetics; PD, pharmacodynamics; NDA, new drug application; IND, investigational new drug.


