
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Phase 0 Clinical Trials in Cancer Drug Development: From FDA Guidance to Clinical Practice
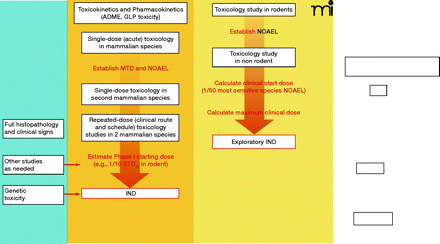
Schema outlining the preclinical testing strategy for traditional and exploratory INDs. Under the traditional IND, for other therapeutic areas, safety pharmacology studies are conducted to address the systemic effects of a new agent on vital cardiovascular, CNS, and respiratory systems; these studies can be conducted as stand-alone trials or as a component of later animal studies. The FDA may not require the conduct of safety pharmacology studies under an exploratory IND if the study involves a single microgram-quantity dose for imaging or PK analysis. In oncology, safety pharmacology studies are not required prior to filing of an IND, exploratory or traditional. Other studies generally conducted under a traditional IND include pharmacokinetic and pharmacology studies to evaluate different dosing schedules or routes of administration, such as tests of animal absorption, distribution, metabolism, and excretion (ADME), analytical assays, and drug formulation testing. Both INDs require single-dose (acute) toxicology studies in two mammalian species to determine toxic and safe doses. For the exploratory IND, these studies may have a single dose or dose escalation design; subsequent repeat-dose toxicology studies in oncology reflect the proposed clinical schedule. For the traditional IND, two mammalian species are tested for cumulative toxicities arising from repeat-dosing that reflects the clinical route and schedule, including full histopathology and clinical sign evaluation; other preclinical studies to assess drug or vehicle activity and safety and support the IND can be conducted as needed. Genetic toxicity testing of oncology agents is not necessary until before Phase II testing. Full genetic toxicity evaluation includes in vitro bacterial reverse mutagenicity tests, in vitro mammalian chromosome damage tests, and in vivo chromosomal damage tests (i.e., in vivo rodent micronucleus assay). MTD, maximum tolerated dose; NOAEL, no observed adverse effect level; 1/10 STD10, one-tenth of that dose which causes severe toxicity or death (STD) in 10% of animals; GLP, good laboratory practices.


