|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2017 | Volume
: 5
| Issue : 1 | Page : 27-33 |
|
Role of volatile organic compounds from gloss paint, emulsion, and thinner on the testes of wistar rats (Rattus norvegicus)
Adeoye Oyetunji Oyewopo1, Joseph Babatunde Dare2, Olugbemi Tope Olaniyan3, Akunna Godson Gabriel4
1 Department of Anatomy, University of Ilorin, Ilorin, Nigeria
2 Department of Anatomy, Bingham University, Karu, Nasarawa, Nigeria
3 Department of Physiology, Bingham University, Karu, Nasarawa, Nigeria
4 Department of Anatomy, Federal University Ndufu-Alike Ikwo, Ebonyi, Nigeria
| Date of Web Publication | 11-Jul-2017 |
Correspondence Address:
Adeoye Oyetunji Oyewopo
Department of Anatomy, University of Ilorin, Ilorin
Nigeria
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/2315-7992.210252

Introduction: Volatile organic compounds (VOCs) in paint are considered harmful to the environment, especially for people who work with them on a regular basis. Aim: In this study, we investigated the effect of VOCs in gloss paint, emulsion, and thinner on the testes of male Wistar rats. Materials and Methods: Twenty adult Wistar rats (100–200 g) were assigned to four (4) groups (A–D) of five rats each. Groups A (thinner), B (emulsion paint), and C (gloss paint) were exposed to fumes from one coating of an improvised chamber for 1 h daily for 21 days while Group D was the control group exposed to fresh air. The rats were exposed to the test chemicals using an improvised chamber of a carton with dimensions 37 cm × 25 cm × 25 cm and had a cross-ventilation (aeration) with six triangular holes of base 4 cm with spaces approximately 1 cm apart and 2 cm on each side. The rats were usually brought out of the animal house, placed in the carton coated with their respective paints for a period of 1 h daily, and then returned to the animal house under normal standard room temperature for 21 days. The rats were sacrificed 24 h after the last exposure day, by cervical dislocation. Results: In this study, the t-test for the body weight of the animal showed no statistical significance (P > 0.05). There was a significant decrease in sperm count and motility and deranged testicular profile in the groups exposed to nitrocellulose thinner, emulsion paint, and gloss paint. The follicle-stimulating hormone (FSH) values increased from those exposed to gloss paint, control thinner, and emulsion. The luteinizing hormone (LH) values increased from emulsion, control, gloss, and thinner. The testosterone (TT) values increased from gloss, emulsion, control, and thinner. Conclusion: We concluded that the exposure to VOCs present in paint has a deleterious effect on the reproductive potentials of an adult male Wistar rat. Keywords: Infertility, organic compounds, paints, paint thinner, testes, Wistar rats
How to cite this article:
Oyewopo AO, Dare JB, Olaniyan OT, Gabriel AG. Role of volatile organic compounds from gloss paint, emulsion, and thinner on the testes of wistar rats (Rattus norvegicus). Ann Bioanthropol 2017;5:27-33 |
How to cite this URL:
Oyewopo AO, Dare JB, Olaniyan OT, Gabriel AG. Role of volatile organic compounds from gloss paint, emulsion, and thinner on the testes of wistar rats (Rattus norvegicus). Ann Bioanthropol [serial online] 2017 [cited 2018 Jul 8];5:27-33. Available from: http://www.bioanthrojournal.org/text.asp?2017/5/1/27/210252 |
| Introduction | |  |
Paint can be defined as any liquid, of liquefiable or mastic composition, which after application to a substrate in a thin layer, is converted to a solid film.[1] It is used mostly for protection, color, or provision of texture to objects. It is mostly applied as liquid, which then dries to solid. Human beings are exposed to paint every day at home, places of work, or daily activities (paint used for walls, windows, doors, furniture, cars, and major things we make use of in our daily life), not taking cognizance of its effects.
Paints are classified commonly into two major types: Oil-based paint and water-based paint. In general, water-based paints are made of volatile organic compounds (VOCs), can be easily cleaned up with water, have an elastic, flexible finish resistant to cracking and quick drying, and a stable color over time without yellowing.[2] In contrast to the water-based paint, oil-based paint requires cleanup with mineral spirits, tends to have more VOCs, has attractive gloss, good leveling (brush strokes fill in in to create a smooth finish), and is hard and durable.[3] The binder, commonly called the vehicle, is the film-forming component of the paint, one of the active components that must be present. Synthetic or natural resins such as alkyds, acrylics, vinyl acetates, polyesters, melamine resins, epoxies, or oils were also included.[4]
Gloss finish paint is generally more resistant to damage than flat paint (eggshell gloss); it becomes glossier through burnishing or staining with grease. However, glossy paint may lose its gloss and scratched if abraded. It is used in traditional household interiors; walls are usually painted in flat or eggshell gloss, wooden trim (including doors and window sash) in high gloss, and ceilings almost invariably in flat paint. Similarly, the exterior trim is usually painted with high quality gross while the body of the house is painted in gloss of lower quality.[5]
An emulsion is a mixture of two or more liquids that are normally immiscible.[4] It is a colloid in which both phases are liquids and oil in water emulsion. Emulsion paint also refers to a type of paint in which the pigment is suspended in a vehicle, usually a synthetic resin, which is dispersed in water as an emulsion.[4] Paint thinner is a solvent used to thin oil-based paints or clean up after their use. Commercially, solvents labeled “paint thinners” are usually mineral spirits having a flash point at about 40°C (104°F), the same as some popular brands of charcoal starter.[4] Solvents used as thinners include: Acetone mineral turpentine naphtha, methyl ethyl ketone, di ethyl formo amide 2-butoxyethanol, or any other glycol ethers, ethylbenzene, xylene, n-butyl acetate butan-1-ol, and others.[4]
VOCs are emitted as gases from certain solids or liquids; they include a variety of chemicals, some of which may have short-term and long-term adverse health effects. The concentrations of many VOCs are constantly higher indoors (up to 10 times higher) than outdoors;[5] a VOC is emitted by a wide array of products numbering in the thousands. Examples include paints and lacquers, strippers, cleaning supplies, and pesticides.[6] Paints, varnishes, and wax all contain organic solvents as do many cleaning, disinfecting, cosmetic, and hobby products. Some of the constituents of paint are hazardous to health.[7] VOCs in paints are considered to be harmful to the environment, especially for people who work with them on a regular bases.[8]
| Materials and Methods | |  |
Animals
A total of 20 male Wistar rats weighing 100–200 g were used for the study. The rats were given standard rat pellets diet, water, and kept in the Animal Holdings of the Department of Anatomy, Faculty of Basic Medical Science of the University of Ilorin in an ideal sanitary condition for the entire period of the experiment.
Chemicals
The gloss paint, thinner, and emulsion of Princess Acrylic Supertex, made in Nigeria by YSR Company Limited ® No. CT-3965, purchased from a store that was “a registered dealer” at No. 4, Muritala Mohammed Way, Ilorin, Kwara in Nigeria, opposite Sterling Bank. Thinner in Group A, emulsion paint in Group B, and gloss paint in Group C were inhaled by the experimental animals while group D was the control group exposed to fresh air only.
The research work was conducted in conformity with the rules and guidelines of the Animal Ethics Committee of the University of Ilorin in the years 2013 and 2014.
Methodology
Twenty adult Wistar rats (100–200 g) were assigned into four groups (A–D) of five rats each. The rats were acclimatized for 2 weeks, after which Group A rats were exposed to thinner, Group B rats were exposed to emulsion paint, Group C rats were exposed to gloss paint while Group D rats served as the control and were exposed to fresh air; the exposure lasted for 1 h daily for 21 days.
The rats were exposed to the test chemicals using an improvised chamber of a carton with dimensions 37 cm × 25 cm × 25 cm and had a cross-ventilation (aeration) with six triangular holes of base 4 cm with spaces approximately 1 cm apart and 2 cm on each side. The rats were usually brought out of the animal house, placed in the carton coated with their respective paints for a period of 1 h daily, and then returned to the animal house under normal standard room temperature for 21 days.
Animal sacrifice
The rats in each group were sacrificed 24 h after the last exposure day, by cervical dislocation, after which the limbs were pinned to a board and medial incision was done using a surgical blade, scalpel, and forceps. Blood samples were collected from each rat through the apex of the heart with a needle and syringe into a labeled anticoagulant (heparin) bottle for centrifugation at 2,000 revolutions for 15 min, which was then transferred to the chemical pathology laboratory for an assay of the follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels. The caudal epididymis was also collected and placed in normal saline for semen analysis using Neubauer-improved hemocytometer manufactured by Marienfeld, Germany. The right testis was removed and it was immediately fixed in a labeled bottle containing fixative (10% buffered formal saline) for 24 h before tissue processing.
Sperm analysis
The testes from each rat were carefully exposed and removed. They were trimmed free of the epididymis and adjoining tissues. The caudal part of the epididymis was removed and placed in a beaker containing 1 mL of physiologic saline solution. Each section was quickly macerated with a pair of sharp scissors and left for a few minutes to release its spermatozoa into the saline solution. Sperm motility, count, progressivity, morphology, and viability (ratio of motile to nonmotile) was determined.[9] Semen drops were placed on the slide and two drops of warm 2.9% sodium citrate were added. The slide covered with a cover slip and examined under Olympus compound microscope (X5Z- 107BN, Tokyo, Japan) using X40 magnification for percentage motility. Sperm count was done under the microscope using Neubauer-improved. The spermatozoon was considered of abnormal morphology if it had a rudimentary tail, round head, or detached tail and was expressed as a percentage of morphologically normal sperm. They were also graded as C (fair directional forward movement) and B (good directional forward movement) according to the World Health Organization (WHO) standard.
Histological analysis
The testes were fixed in Bouin's fluid for at least 24 h, after which these underwent dehydration in ascending grades of alcohol 50%, 70%, and 90%, and absolute alcohol I and II; xylene was used as the clearing agent (two changes). Infiltration and embedding in paraffin wax (56°C Mpt) were done in the oven at 60°C, and the tissues were blocked out in paraffin wax. Serial sections were obtained using rotary microtome at 3 micron, mounted and dewaxed using hot plate on albumenized slide; the tissues were stained with hematoxylin and eosin stains after which they were passed through a mixture of equal concentration of xylene and alcohol and covered with cover slip using Canada balsam. The photographs of the tissue were taken through a light microscope (Olympus compound microscope (X5Z- 107BN, Tokyo, Japan) with a digital camera (Fujifilm, 8.2 megapixels, Tokyo, Japan) by mounting the digital camera directly on the eyepiece.
Determination of biochemical parameters
Serum hormonal assays–luteinizing hormone and follicle-stimulating hormone
The assays were done according to the procedure adapted by Amballi et al.[10] Briefly, the blood that was collected into plain containers was allowed to clot. Each sample was centrifuged at 1,000 rpm for 10 min to achieve separation. The serum obtained was put into aliquots in each case, labeled, and stored at -20°C. One aliquot of each specimen was taken at a time to avoid repeated freezing and thawing, and the samples were analyzed for hormone estimation using enzyme immunoassay (EIA) according to WHO-matched reagent program protocol (manual) for EIA kits (protocol/version of December 1998 for LH, FSH). The kits were supplied by National Institute of Diabetes and Digestive and Kidney Diseases-National Institutes of Health (USA).
Determination of serum testosterone concentrations
Testosterone (TT) concentrations in plasma were determined by the enzyme immunoassay technique based on the principle of competitive binding between TT and TT-horseradish peroxidase conjugate for a constant amount of rabbit anti-TT, as previously described (Tietz, 1995). Briefly, goat antirabbit immunoglobin G (IgG)-coated wells were incubated with TT standards, controls, samples (blood sera and supernatants of testicular homogenates), TT-horseradish peroxidase conjugate reagent, and rabbit anti-TT reagent at 37°C for 90 min. Unbound TT peroxidase conjugate was removed and the wells were washed. Tetramethylbenzidine was added and incubated, resulting in the development of a blue color. The color development was stopped with the addition of 1N hydrochloric acid, and the absorbance measured spectrophotometrically at 450 nm. A standard curve was obtained by plotting the concentration of the standard versus the absorbance and TT concentrations calculated from the standard curve.
Estimation of morphometric parameters
Histological slides were prepared from the buffered formol saline-fixed testes. However, before embedding, it was ensured that the sections were orientated perpendicular to their long axes, and chosen as “vertical sections.” For each testis, five vertical sections from the polar and equatorial regions were sampled,[11] and an unbiased numerical estimation of the following morphometric parameters was estimated using a systematic random scheme.[12]
Diameter (D) of seminiferous tubules
The diameter of seminiferous tubules with profiles that were round or nearly round were estimated for each animal and a mean, D, was determined by taking the average of two diameters, D1 and D2 (perpendicular to one another). The value was take if D1/D2 ≥ 0.85.
Cross-sectional area (AC) of the seminiferous tubules
The cross-sectional areas of the seminiferous tubules were estimated from the formula AC = π D2/4, (where π is equivalent to 3.142 and D is the mean diameter of the seminiferous tubules).
Number of profiles of seminiferous tubules in a unit area of testis (NA)
The number of profiles of seminiferous tubules per unit area was determined using the unbiased counting frame anticipated by Gundersen.[13] Using this frame, in addition to counting profiles completely inside the frame, we counted all profiles with any part inside the frame provided they did not intersect the forbidden line (full-drawn line) or exclusion edges or their extension.
Numerical density (NV) of seminiferous tubulesNumber of profiles of seminiferous tubules in a unit
This is the number of profiles per unit volume and was determined by using the modified Floderus equation: NV = NA/(D + T)[14] where NA is the number of profiles per unit area, D is the diameter, and T the average thickness of the section.
The evaluation of the diameter was done with a calibrated eyepiece and stage grids (Tokyo, Japan) mounted on a light research microscope (Tokyo, Japan). Estimation of volume density of testicular components and number of seminiferous tubules were done on a computer monitor onto which a graph sheet was superimposed and on which the slides were projected from a research light microscope (Model N -400ME, CEL-TECH Diagnostics, Hamburg, Germany).
Statistical analysis
The results were analyzed statistically by application of Student's t-test, and presented as mean ± standard error of the mean (SEM) with determination of level of significance. This also involved the use of the Statistical package Sciences (SPSS) 16.0 version (Cary, NC, USA). and analysis of variance (ANOVA) to determine the significant differences between the groups at P < 0.05. The analysis and comparison were evaluated for significance at 5% (α = 0.05).
| Results | |  |
Gross anatomical parameters
In this study, the t-test for the body weight of the rats showed no statistical significance (P > 0.05). In contrast, Group A and Group C rats had a significant reduction (P < 0.05) in testicular weight and volume when compared to that of the control group. However, there was no significant change in the testicular weight and volume of Group B rats when compared to that of the control group [Table 1]. | Table 1: Effects of gloss, emulsion, thinner, and fresh air on body weight, testis weight, and volume of Wistar rats
Click here to view |
Effects of paint, emulsion, nitrocellulose thinner, and fresh air (control) on epididymal sperm characteristics
There was a significant decrease (P < 0.05) in sperm motility, normal morphology, count, and viability of Group A, B, and C rats when compared to that of Group D [Table 2]. | Table 2: Effects of gloss paint, emulsion, nitrocellulose thinner, and fresh air (control) on sperm count, sperm motility, normal sperm morphology, progressivity, and life and death ratio of spermatozoa
Click here to view |
Effects of paint, emulsion, nitrocellulose thinner, and fresh air (control) on testicular serum testosterone, follicle-stimulating hormone, and luteinizing hormone
There was a significant decrease in the activity level of FSH in Group C rats. However, there were no significant changes in the activity level of FSH in Groups A and B when compared to the control group. The activity level of LH in all the exposed group was similar to the value seen in the control group. The serum TT level decreased significantly (P > 0.05) in rats exposed to emulsion and gloss paint when compared to those in the control group [Table 3]. | Table 3: Effects of paint, emulsion, nitrocellulose thinner, and fresh air (control) on testicular serum testosterone, follicle-stimulating hormone, and luteinizing hormone
Click here to view |
Testicular morphometry
In this study, there was a significant (P < 0.05) reduction in the mean seminiferous tubular diameters, cross-sectional area, number of profiles per unit area, and the mean numerical density of seminiferous tubules of rats exposed to gloss paint and thinner when compared to the control group [Table 4]. However, there was no significant difference on the morphometric values of rats exposed to emulsion and the control groups of rats [Table 4]. | Table 4: Effects of paint, emulsion, nitrocellulose thinner, and fresh air (control) on seminiferous tubular diameter (μm), cross.sectional area Ac (×103 μm2), numerical densities of tubules NA (×10-8 μm-2), and no. of profiles per unit area NV (×10-8 μm-3)
Click here to view |
Histology of the testes and epididymis
In this study, the exposed rats showed destructive changes in their seminiferous tubular epithelium, interstitial tissues and epididymal lining [Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5], [Figure 6], [Figure 7], [Figure 8]. Their profiles were characterized by hypospermatozoa formation in the tubules when compared to the control group of rats. The tubular epithelium and epididymis of the group of rats exposed to emulsion [Figure 3], [Figure 4] exhibited greater pathological alterations when compared to the groups of rats exposed to gloss paint and thinner.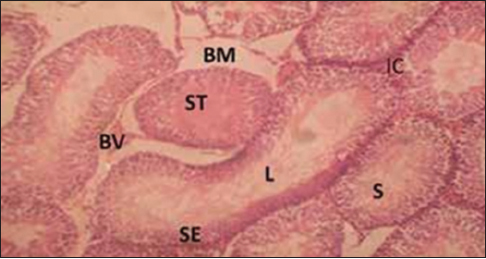 | Figure 1: Cross-section of the testis of group for the control group (for 3 weeks) Stain: Hematoxylin and eosin, Magnification: ×200, BV = Blood vessel, ST = Seminiferous tubules, IC = Interstitial cells, S = Spermatozoa, L = Lumen, SE = Spermatogenic epithelium, BM = Basement membrane
Click here to view |
 | Figure 2: Cross-section of the testis of group that inhaled gloss paint3 weeks) (for Stain: Hematoxylin andeosin,Magnification: ×200, 3 weeks) Stain: Hematoxylin and eosin, Magnification: ×200, ST = Seminiferous tubules, IC = Interstitial cells, S = Spermatozoa, L = Lumen, SE = Spermatogenic epithelium
Click here to view |
 | Figure 3: Cross section of the testis of the group that inhaled Nitrocellulose thinner (for 3 weeks). Stain: Hematoxylin and eosin. Magnification: ×200. ST = Seminiferous tubule, L = Lumen, S = Spermatozoa, SE = Spermatogenic epithelium
Click here to view |
 | Figure 4: Cross section of the tests of the group that inhaled emulsion paint (for 3 weeks). Stain: Hematoxylin and eosin, Magnification: ×200. ST = Seminiferous tubule, S = Spermatozoa, L = Lumen, SE = Spermatogenic epithelium
Click here to view |
 | Figure 5: Cross section of the epididymis of the control group (for 3 weeks) Stain: Hematoxylin and Eosin, Magnification: ×200. S = Spermatozoa, B = Basal cells, C = Columnar cells, DE = Duct of the epididymis
Click here to view |
 | Figure 6: Cross section of the epididymis of the group that inhaled gloss paints (for 3 weeks). Stain: Hematoxylin and eosin. Magnification: ×200. S = Spermatozoa, B = Basal cells, C = Columnar cells
Click here to view |
 | Figure 7: Cross-section of the epididymis of group that inhaled nitrocellulose thinner (3 weeks). Stain: Hematoxylin and eosin, Magnification: ×200, S = Spermatozoa, BV = Blood vessels, C = Columnar cells, DE = Duct of the epididymis
Click here to view |
 | Figure 8: Cross section of the epididymis of the group that inhaled emulsion paint (for 3 weeks). Stain: Hematoxylin and eosin. Magnification: ×200. S = Spermatozoa, BV = Blood vessels, C = Columnar cells
Click here to view |
| Discussion | |  |
Irrespective of the infertility status, a strong correlation between house hold chemicals and sperm dysfunction has been established.[15] In this study there was a significant decrease in testicular weight and volume of rats exposed to paint, emulsion and thinner. The result in this study conform to previous reports of significant reduction in gross anatomical parameters due loss in testicular integrity as a result of various oxidative derangements.[16]
The decrease in testicular weight could have been as a result of Sertoli cell apoptosis due to oxidative stress, hence establishing the association between the testis size and spermatogenesis. More Sertoli cells More Details means more germ cells per testis, and the number of sertoli cells per gram of tissue combined with the number of spermatids per Se rtoli cells is associated with sperm production per gram of testis.
While the representative sections of the histomorphometric features of the testes of control rats were fairly normal with a largely preserved seminiferous epithelium, exposed rats (Group B, C, and D) portrayed evidences of degenerative changes in their seminiferous epithelium characterized by decrease in the geometric values, hypospermatozoa formation, interstitial edema, and vacuolization of the interstitium. Our histological findings are in concordance with a report that chronic exposure to solvents (thinner) was noted to cause loss of weight in the prostate and testis and predisposition to fertility problems.[16],[17]
In agreement with previous reports,[18],[19] the sperm count and sperm motility of the control group demonstrated an increase of 65.20 × 106 mL and 35.60% in values, respectively, when compared to the groups exposed to nitrocellulose thinner that demonstrated an increase of 35.20 × 106 mL and 32%, emulsion paint that demonstrated an increase of 38.40 × 106 mL and 36.40%, and gloss paint that demonstrated an increase of 42.50 × 106 mL and 40.20% in the sperm count and sperm motility, respectively.
Reactive oxygen species (ROS) is essential for proper sperm function through intracellular signal transduction where it encourages capacitation, the acrosome reaction, and oocyte attachment.[20],[21] The testicular impairments evidenced in this study could have been as a result of oxidative damage induced by the exposed materials. Usually, there is a balance in the seminal plasma between ROS production and removal but exposure to hazardous materials can lead to an excess production of ROS.[19]
| Conclusion | |  |
The current study revealed that exposure to VOCs present in paint causes testiculotoxic and spermatotoxic effects in rats. This is based on the evidence by the alterations in the andrological, biochemical, and geometrical features.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| References | |  |
| 1. | Schneck FX, Bellinger MF. Abnormalities of the testes and scrotum and their surgical management. In: Wein AJ, editor. Campbell-Walsh Urology. 9 th ed. Philadelphia: Saunders; 2007. p. 120-7.  |
| 2. | Pappa S. Oldest Human Paint-Making Studio. Increased cytogenic damage in outdoor paints. Livescience; 2011. p. 5-7.  |
| 3. | Karimov KhIa, Dadazhanov ShN, Gil'dieva MS. Rat reproductive cells as biological indicators of the effect of environmental factors. Morfologiia 2003;123:69-71.  |
| 4. | Berbee ML, Taylor JW. Dating the molecular clock in fungi- how close are we? Fungal Biol Rev 2010;24:1-16.  |
| 5. | Berendsen AM. Marine painting manual. Graham and Trotman. London: Springer Netherlands; 1989. p. 113-4.  |
| 6. | Behr A, Johnen L. Myrcene as a natural base chemical in sustainable chemistry: A critical review. ChemSusChem 2009;2:1072-95.  [ PUBMED] |
| 7. | Spurgeon A. Watching paint dry: Organic solvent syndrome in late-twentieth-century Britain. Med Hist 2006;50:167-88.  [ PUBMED] |
| 8. | Stoye D, Funke W, Hoppe L, Hasselkus LG, Curtis K, Hoehne HJ, et al. Paints and Coatings in Ullmann's Encyclopedia of Industrial Chemistry. Weinheim, Germany: Wiley-VCH, 2006. p. 1327-30.  |
| 9. | Saalu LC, Togun VA, Oyewopo AO, Raji Y. Artificial cryptorchidism and the moderating effect of melatonin in sprague-Dawley rats. J Med Sci 2006;6:2889-94.  |
| 10. | Amballi AA, Dada OA, Adeleye AO, Jide S. Evaluation of the determination of reference ranges for reproductive hormones (prolactin, FSH, LH, and testosterone) using enzyme immuno assay method. Sci Res Essays2007;2:135-8.  |
| 11. | Qin DN, Lung MA. Morphometric study on Leydig cells in capsulotomized testis of rats. Asian J Androl 2002;4:49-53.  [ PUBMED] |
| 12. | Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its predictions. J Microsc 1987;147:229-63.  [ PUBMED] |
| 13. | Gilliland KO, Freel CD, Lane CW, Fowler WC, Costello NJ. Multilamellar bodies as potential scattering particles in human age-related nuclear cataracts. Mol Vis 2001;7:120-30.  |
| 14. | Akunna GG, Saalu LC, Ogunlade B, Akingbade AM, Anderson LE, Olusolade FS. Histo-morphometric evidences for testicular derangement in animal models submitted to chronic and sub-chronic inhalation of fragrance. Am J Res Commun 2015;3:84-101.  |
| 15. | Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ 1992;305:609-13.  [ PUBMED] |
| 16. | Sofikitis N, Miyagawa I. Experimental models for the study of varicocele: A selected review. Jpn J Fertil Steril 1992;38:168-77.  |
| 17. | Marmar JL, Agarwal A, Prabakaran S, Agarwal R, Short RA, Benoff S, et al. Reassessing the value of varicocelectomy as a treatment for male subfertility with a new meta-analysis. Fertil Steril 2007;88:639-48.  [ PUBMED] |
| 18. | Naughton CK, Nangia AK, Agarwal A. Pathophysiology of varicoceles in male infertility. Hum Reprod Update 2001;7:473-81.  [ PUBMED] |
| 19. | de Lamirande E, Gagnon C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects. Hum Reprod 1995;10(Suppl 1):15-21.  [ PUBMED] |
| 20. | de Lamirande E, Lamothe G. Reactive oxygen-induced reactive oxygen formation during human sperm capacitation. Free Radic Biol Med 2009;46:502-10.  [ PUBMED] |
| 21. | Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 1987;81:459-69.  [ PUBMED] |
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5], [Figure 6], [Figure 7], [Figure 8]
[Table 1], [Table 2], [Table 3], [Table 4]
|