|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2011 | Volume
: 17
| Issue : 1 | Page : 7-12 |
| |
Evaluation of smoking genotoxicity in Turkish young adults
Ayse G Zamani1, H Gul Durakbasi-Dursun2, Sennur Demirel2, Aynur Acar1
1 Department of Medical Genetics, University of Selšuk, Meram Medical Faculty, Konya, Turkey
2 Department of Medical Biology, University of Selšuk, Meram Medical Faculty, Konya, Turkey
| Date of Web Publication | 18-Jun-2011 |
Correspondence Address:
Ayse G Zamani
Nalcaci Cad. Saglik Apt., No:5/5 42060, Selcuklu, Konya
Turkey
 Source of Support: Scientific Research and Project Coordinator of Selcuk
University (Project number: 98/053), Conflict of Interest: None  | 3 |
DOI: 10.4103/0971-6866.82186

 Abstract Abstract | | |
Background: For the past few decades, it has been widely known in developed countries that tobacco is dangerous, but it is still insufficiently realized how big these dangers really are.
Aims: To determine and evaluate micronuclei (MN) frequencies of young smokers and nonsmokers in three different tissues (peripheric blood lymphoctes, buccal mucosa, and exfoliative urothelial cells) at the same time.
Materials and Methods: MN assay was performed on buccal mucosa, urothelial cells, and peripheric blood lymphocyte samples obtained from 15 healthy male smokers (>5 pack-years) and 15 healthy male nonsmoker controls who had not been exposed to any known genotoxic agent.
Statistical Analysis Used: The statistical differences between smoker and nonsmoker groups were calculated by using student t test. The differences between smoker-group tissues were compared by ANOVA.
Results: It was found that MN frequency (mean value ± standard deviation) in oral mucosa cells from smokers and controls were 1.20 ± 0.22% and 0.26 ± 0.10%; in urothelial exfoliative cells, 1.29 ± 0.28% and 0.12 ± 0.08%; in peripheric blood lymphocytes, 1.53 ± 0.23% and 0.38 ± 0.12%, respectively. The mean MN frequencies in buccal mucosa, urothelial exfoliative cells, and peripheric blood lymphocytes were significantly higher in smokers than in those of controls (P<0.05). All tissues were affected from smoking, but the most destructive effect was seen in urothelial cells of smokers (P<0.05).
Conclusions: Our data showed that cigarette smoke is a DNA damage causitive agent on exfoliative buccal mucosa and urothelial cells and peripheric blood lymphocytes of young smokers, but it has most destructive effect on urothelial cells.
Keywords: Buccal mucosa, genotoxicity, human lymphocytes, micronucleus, urothelial cell
How to cite this article:
Zamani AG, Durakbasi-Dursun H G, Demirel S, Acar A. Evaluation of smoking genotoxicity in Turkish young adults. Indian J Hum Genet 2011;17:7-12 |
How to cite this URL:
Zamani AG, Durakbasi-Dursun H G, Demirel S, Acar A. Evaluation of smoking genotoxicity in Turkish young adults. Indian J Hum Genet [serial online] 2011 [cited 2016 May 13];17:7-12. Available from: http://www.ijhg.com/text.asp?2011/17/1/7/82186 |
 Introduction Introduction | |  |
In the nineteenth century, tobacco was chewed or inhaled in the form of snuff. The practice of inhaling became popular with introduction or mass marketing of blended cigarettes in 1913. [1],[2] Consumption of cigarette smoking increased from less than 5 billion cigarettes per year in 1905, to more than 90 billion in 1925 and to almost 600 billion in 1960s in U.S. [3] By the year 2030, estimated deaths from tobacco use will be 10 billion worldwide, annually. [4]
It is known that smoking is responsible for a substantial number of human health problems. Cigarette smoke is made up of thousands of chemicals and some of them are known to be mutagens and carcinogens. [5] Cytogenetic markers such as chromosome aberration assay, sister-chromatid exchange assay, and micronucleus (MN) assay have been widely used as an indicator of genotoxic effects. [6] But MN assay is the most sensitive and simple. Since the cytogenesis block method was developed in human lymphocytes, many laboratories have used this methodology both in vitro and in vivo studies. [7] This assay was adapted to exfoliated human cells by Stich and Rosin. [8] MN which are detectable in exfoliated cells of mouth, lung, bladder, and cervix seem to reflect genotoxic effect in the proliferating basal layers. [8]
In this present study, we aimed to determine and evaluate MN frequencies of smokers and nonsmokers in three different tissues (peripheric blood lymphocytes, buccal mucosa and exfoliative urothelial cells) at the same time.
 Materials and Methods Materials and Methods | |  |
Planned study population, including controls
This study was carried out in volunteer students of Meram Medical Faculty, Selcuk University. The volunteers were interviewed about their smoking habits, recent illnesses, use of drugs, exposure to hazardous agents, and other possible confounding problems. Qualified 15 healthy male smokers and 15 healthy male nonsmokers were matched for age (P>0.05). The smokers were asked about when they began smoking and how many packs they consumed daily. They were included into the study if they had smoked more than 1 pack per day for more than five years (>5 pack-years). All the volunteers in this study signed the Informed Consent Form.
Methods of Sampling Procedure
Sampling of peripheric blood lymphocytes
Heparinized blood samples were obtained from the subjects of both groups, and standard lymphocyte cultures were performed. For the MN assay, cytochalasin B at final concentration of 3 μg/ml was added to 44 th hour of the culture of lymphocytes, according to the method of Fenech and Morley. [7] At the end of the incubation period, cultures were harvested. First, they were treated with prewarmed hypotonic solution (0.075 M KCl) for a few minutes at room temperature and then resuspended twice in cold fresh fixative (methanol: glacial acetic acid, 3: 1). Fixed cells were dropped onto cold microscope slides and air-dried.
Sampling of urothelial cells
Urine samples were processed within 4 hours of voiding and cells were collected by centrifugation of second morning void (150-200 ml), as described previously. [9] Pelleted cells were dispersed into the fresh fixative. After 25 minutes, the cell suspension was centrifuged and resuspended in 0.5 ml fresh fixative and then dropped onto precleaned microscope slides. They were allowed to air-dry.
Sampling of buccal mucosa cells
Oral mucosa of the inner side of the lower lip and left and right lower part of the cheek was swabbed with a moistened wooden tongue depressor. [10] The cells were transferred directly onto precleaned microscope slides and allowed to dry. Within one week of sampling, slides were placed in fresh fixative for 15 minutes.
Staining procedure
Peripheric blood lymphocytes were stained in 5% Giemsa solution for 5 to 7 minutes. Buccal mucosa and urothelial cells were stained by the Fuelgen reaction and counterstained with Fast-Green according to following procedure: 1 M HCl, 1 minute at room temperature; 1 M HCl, 10 minutes at 63°C; after cooling the slides for 15 to 20 minutes, 1 M HCl, 5 minutes at room temperature; rinse in distilled water for 15 to 20 minutes; placed in Schiff's reagent for 2.5 to 3 hours; rinse in running top water for 15 to 20 minutes. The preparations were placed with 1% Fast-Green for 10 to 20 seconds, washed three times in absolute ethanol, and air-dried.
Scoring procedure
Slides were coded and scored blind under a magnification of 1 00 X. Two scorers were used to analyze 2 000 binucleated cells (1 000 for each scorer) per subject. Established criteria were followed for estimating the frequency of micronucleated cells. [11],[12]
Methods of statistical analysis
The statistical differences between the smoker and nonsmoker groups were calculated by using student t test. The mean values were given as mean ± standard deviation and the value of P<0.05 was considered as significant.
The differences between smoker groups were compared by ANOVA. Additionally, the value being less than 0.05 was considered as significant.
 Results Results | |  |
The induced MN frequencies in buccal mucosa, lymphocytes and urothelial cells of smokers and nonsmokers are summarized in [Table 1] and [Table 2], respectively. MN frequencies (mean value ± SD) of nonsmokers and smokers were averaged and found to be 1.20 ± 0.22% and 0.26 ± 0.10% in buccal mucosa cells, 1.29 ± 0.28% and 0.12 ± 0.08% in urothelial exfoliative cells, and 1.53 ± 0.23% and 0.38 ± 0.12 in peripheric blood lymphocytes. The mean MN frequencies in buccal mucosa, urothelial exfoliative cells, and peripheric blood lymphocytes were significantly higher in smokers than in those of controls (P<0.05) [Table 3].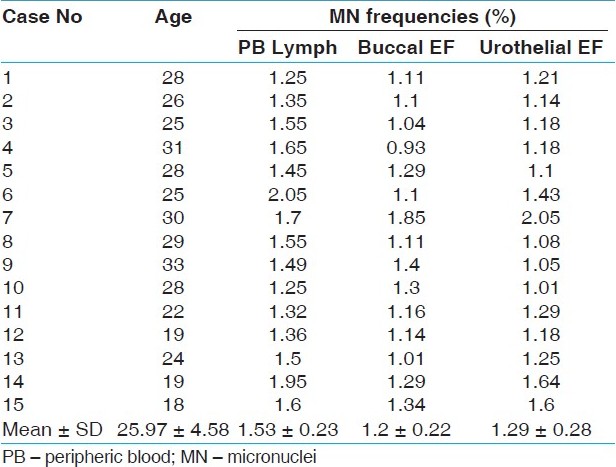 | Table 1: MN data obtained from PB lymphocytes and exfoliated cells of the smokers
Click here to view |
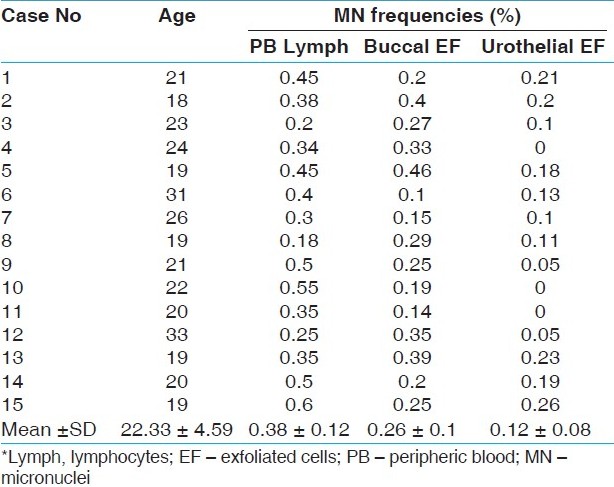 | Table 2: MN data obtained from PB lymphocytes and exfoliated cells of the nonsmoker control group
Click here to view |
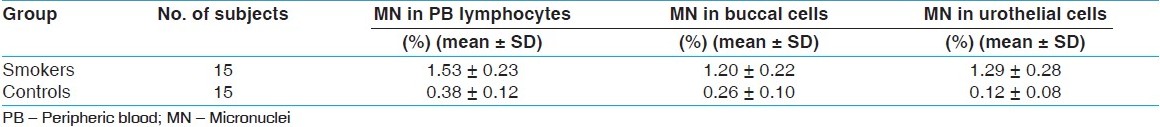 | Table 3: Comparison of the mean MN frequencies in lymphocytes and exfoliated cells of smokers and controls (P<0.05).
Click here to view |
We compared MN frequencies of buccal, urothelial, and peripheric blood lymphocytes of smokers with each other. The percentage of genotoxic effect of smoking on different tissues is given in [Figure 1]. Numbers were given by mean ± s.e.m. which represents the degree of significance between the groups (P<0.05). All tissues were affected from smoking, but the most destructive effect was seen in urothelial cells of smokers [Figure 1].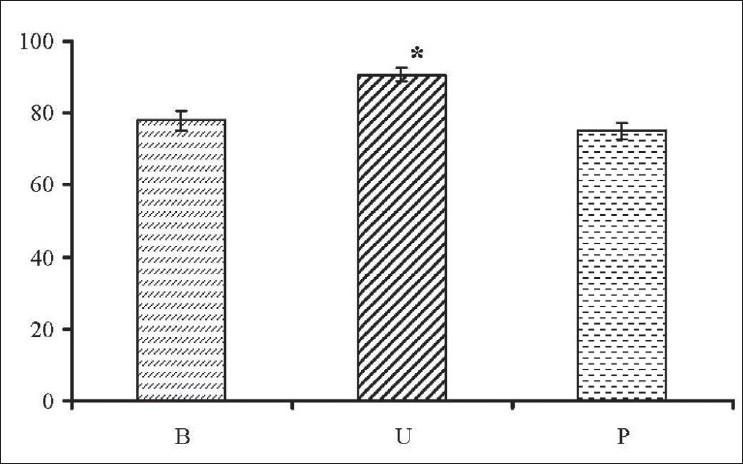 | Figure 1: Comparison of different tissues of smoker group. The percentage of genotoxic effect of smoking on the buccal (B), urethral (U), and peripheric blood (P) tissues. Numbers were given as mean ± s.e.m. * represents the degree of signifi cance between the groups (P<0.05).
Click here to view |
 Discussion Discussion | |  |
MN are small fragments of extranuclear DNA formed during cell division, which provide a nonspecific but quantifiable marker of DNA damage, so it is used to identifiy cellular damage caused by carcinogenic agents. [7] Smoking is a well-known source of carcinogenic influence in humans. More than 4 000 chemical substances, such as acetone, benzene, benzopyrene, cyanamide, methane, which are found in cigarette smoke are carcinogenic. [5] In many studies, MN frequencies in different tissues of cigarette smokers were evaluated separately. We aimed to investigate the genotoxic effect of cigarette smoking by the presence of MN in three different tissues at the same time. So, we evaluated MN frequencies in buccal mucosa, urothelial cells, and peripheric blood lymphocytes in the same study group.
When we compared MN rates of these three different tissues, it was seen that DNA damage in urothelial cells was higher than in buccal mucosa and peripheric blood lymphocytes [Figure 1]. Concerning the urothelial tissue, increased MN levels in exfoliated urothelial cells related to tobacco exposure have also been reported in previous studies; MN rates varied from 4.7 to 9%. [13],[14],[15] In this study, observed MN levels averaged in controls were 0.12 ± 0.08% and 1.29 ± 0.28% in smokers. The percentage of smokers was lower than previous data, but it is meaningful when smokers and nonsmokers compared each other.
Leucher-Michel investigated 13 exsmokers who had stopped smoking for at least 2 years and no significant correlation could be found between postexposure duration and the decrease of MN in exfoliated urothelial cells. This result suggested that no significant decrease of MN rates occurs in the years after cessation of smoking. [13] Peripheric blood lymphocytes may accumulate genetic damage over years before in vitro stimulation. [16] In contrast to lymphocytes, urothelial cells have a rapid turnover; cells are shed continuously from the surface of the epithelium. MN observed in exfoliated cells resulted from DNA damage in the basal cells of epithelium. Stich et al., however, detected a pronounced increase in MN in the oral cells of "reverse "smokers, who hold the lit end of the cigarette in the mouth. [17] The kinetics of MN formation in oral mucosa of irradiated patients showed that micronucleated cell frequencies increased throughout the treatment, then rapidly declined to the values observed in untreated tissues within 1 month of cessation of treatment. [18] Rosin explained this decrease by the fact that all damaged cells have been exfoliated and no more chromosomal breakage is occuring in the basal cells. Comparison of the results of experiments of exfoliated bladder cells with exfoliated mouth cells suggests that bladder cells are more sensitive toward tobacco smoking. [13],[15] On the other hand, MN have been described as inheritable anomalies capable of being the origin of micronucleated clones. [19],[20] This finding is supported by a study with data from the National Bladder Cancer Study (USA); the risk of bladder cancer in former smokers has been shown to remain high, even after smoking cessation. [15] On this basis, high levels of MN in exfoliated urothelial cells of exsmokers could be explained by the presence of clones of remaining micronucleated basal cells; a greater number of exsmokers would have been necessary to evaluate this hypothesis. [13],[21] MN assay with exfoliated cells test reflects damage that occurred in the basal tissue on a small time scale before the sampling. [22]
Tobacco use is one of the chief preventable causes of morbidity and mortality worldwide. Currently, there are about one billion smokers in the world and one of ten deaths among adults is attributable to tobacco use. [23],[24] Another billion young adults are estimated to start smoking by year the 2030 and about 10 million would die due to the habit of tobacco use, 70% occurring in developing countries. [24] Local surveys carried out on Turkish youth demonstrate that 29.6% of girls and 46.4% of boys aged 13 to 14 years have ever smoked and 4.3% and 14.1% of them, respectively, are current smokers. [25] Various studies conducted in youngsters of different ages and regions have indicated that smoking prevalence rates of ever-smokers differ between 0.7 to 21.1% among girls, and 1.1 to 52.4% among boys. [26] In our study, smokers with a smoking history of more than 5 pack-years are possible candidates for bladder cancer which will be developed in the future.
It has been reported in many epidemiological studies and reviews that smoking increases the risk of bladder cancer. [27] Zeegers et al.[28] reported that there was a relation between the period and the amount of smoking. They reported that smoking increased the risk of bladder cancer 3-fold, and this increase was correlated with the number of years that smoking continued and the number of cigarettes smoked in a meta-analysis including 43 case-control cohort studies. [28] It was noticed that duration time period and progressive amount of smoking increased the bladder cancer risk in a pooled analysis including 11 case-control studies. Moreover, the risk increased particularly if the number of smoked cigarettes exceeded 15 to 20 cigarettes per day. [29] In the same study it was shown that this risk decreased after quitting smoking for 1 to 4 years; however, it never fell to the level of nonsmokers even 25 years after quitting. In our study, smokers had smoked more than 1 pack per day for more than five years (>5 pack-year).
As a conclusion, in developing countries like Turkey, smoking is closely connected with bladder cancer. Our data showed that cigarette smoke is a DNA damage causitive agent on exfoliative buccal mucosa and urothelial cells and peripheric blood lymphocytes of young smokers, but it has most destructive effect on urothelial cells. The association between smoking and bladder cancer lasts even after quitting smoking. Therefore, the early education of youth about the harmful effects of smoking on health has an important role in the prevention of this habit.
 References References | |  |
| 1. | Burns DM. Changes in cigarette related disease risks and their implications for preventation and control. Nat Inst Health 1997;4213:13-53. 
|
| 2. | Bilello KS, Murin S, Matthay RA. Epidemiology, etiology, and prevention of lung cancer. Clin Chest Med 2002;23:1-25. 
|
| 3. | Hurt RD, Ebbert JO. Preventing lung cancer by stopping smoking. Clin Chest Med 2002;23:27-36. 
|
| 4. | Peto R, Lopez AD, Boreham J, Thun M, Heath C Jr, Doll R. Mortality from smoking worldwide. Br Med Bull 1996;52:12-21. 
|
| 5. | Gürel E, Orta T, Günebakan S, Utkusavaº A, Kolusayin Ozar MO. Can micronucleus technique predict the risk of lung cancer in smokers? Tuberk Toraks 2005;53:225-30. 
|
| 6. | Titenko-Holland N, Moore LE, Smith MT. Measurement and characterization of micronuclei in exfoliated human cells by fluorescence in situ hybridization with a centromeric probe. Mutat Res 1994;312:39-50. 
|
| 7. | Fenech M, Morley AA. Measurement of micronuclei in lymphocytes. Mutat Res 1985;147:29-36. 
|
| 8. | Stich HF, Rosin MP. Micronuclei in exfoliated human cells as a tool for studies in cancer risk and cancer intervention. Cancer Lett 1984;22:241-53. 
|
| 9. | Stich HF, Rosin MP. Quantitating the synergistic effect of smoking and alcohol consumption with the micronucleus test on human buccal mucosa cells. Int J Cancer 1983;31:305-8. 
|
| 10. | Rosin MP, German J. Evidence for chromosome instability in vivo in Bloom syndrome: Increased numbers of micronuclei in exfoliated cells. Hum Genet 1985;71:187-91. 
|
| 11. | Tolbert PE, Shy CM, Allen JW. Micronuclei and other nuclear anomalies in buccal smears: Methods development. Mutat Res 1992;271:69-77. 
|
| 12. | Countryman PI, Heddle JA. The production of micronuclei from chromosome aberrations in irradiated cultures of human lymphocytes. Mutat Res 1976;41:321-32. 
|
| 13. | Lehucher-Michel MP, Di Giorgio C, Amara YA, Laget M, Botta A. The micronucleus assay in human exfoliated urothelial cells: Effect of smoking. Mutagenesis 1995;10:329-32. 
|
| 14. | Reali D, Di Marino F, Bahramandpour S, Carducci A, Barale R, Loprieno N. Micronuclei in exfoliated urothelial cells and urine mutagenicity in smokers. Mutat Res 1987;192:145-9. 
|
| 15. | Majer BJ, Laky B, Knasmüller S, Kassie F. Use of the micronucleus assay with exfoliated epithelial cells as a biomarker for monitoring individuals at elevated risk of genetic damage and in chemoprevention trials. Mutat Res 2001;489:147-72. 
|
| 16. | Natarajan AT, Obe G. Screening of human populations for mutations induced by environmental pollutants: Use of human lymphocyte system. Ecotoxicol Environ Saf 1980;4:468-81. 
|
| 17. | Stich HF, Parida BB, Brunnemann KD. Localized formation of micronuclei in the oral mucosa and tobacco-specific nitrosamines in the saliva of "reverse" smokers, Khaini-tobacco chewers and gudakhu users. Int J Cancer 1992;50:172-6. 
|
| 18. | Rosin MP. The use of the micronucleus test on exfoliated cells to identify anti-clastogenic action in humans: A biological marker for the efficacy of chemopreventive agents. Mutat Res 1992;267:265-76. 
|
| 19. | Franceschi C. Cell proliferation, cell death and aging. Aging (Milano) 1989;1:3-15. 
|
| 20. | Weinberg RA. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res 1989;49:3713-21. 
|
| 21. | Hartge P, Silverman D, Hoover R, Schairer C, Altman R, Austin D, et al. Changing cigarette habits and bladder cancer risk: A case-control study. J Natl Cancer Inst 1987;78:1119-25. 
|
| 22. | Fenech M, Holland N, Chang WP, Zeiger E, Bonassi S. The HUman MicroNucleus Project-an international collaborative study on the use of the micronucleus technique for measuring DNA damage in humans. Mutat Res 1999;428:271-83. 
|
| 23. | Curbing the epidemic: Governments and the economics of tobacco control. The World Bank. Tob Control 1999;8:196-201. 
|
| 24. | Peto R, Lopez AD. Future worldwide health effects of current smoking patterns. In: Koop CE, Pearsno CE, Schwarz MR, editors. Critical issues in global health. New York: Jossey-Bass; 2001. p. 154-61. 
|
| 25. | Erbaydar T, Lawrence S, Dagli E, Hayran O, Collishaw NE. Influence of social environment in smoking among adolescents in Turkey. Eur J Public Health 2005;15:404-10. 
|
| 26. | Erguder T, Soydal T, Uðurlu M, Cakir B, Warren CW. Tobacco use among youth and related characteristics, Turkey. Soz Pravent 2006;51:91-8. 
|
| 27. | Demirel F, Cakan M, Yalçinkaya F, Topcuoglu M, Altug U. The association between personal habits and bladder cancer in Turkey. Int Urol Nephrol 2008;40:643-7. 
|
| 28. | Zeegers MP, Tan FE, Dorant E, van Den Brandt PA. The impact of characteristics of cigarette smoking on urinary tract cancer risk: A meta analysis of epidemiologic studies. Cancer 2000;89:630-9. 
|
| 29. | Brennan P, Bogillot O, Cordier S, Greiser E, Schill W, Vineis P, et al. Cigarette smoking and bladder cancer in men: A pooled analysis of 11 case-control study. Int J Cancer 2000;86:289-94. 
|
[Figure 1]
[Table 1], [Table 2], [Table 3]
| This article has been cited by | | 1 |
Micronucleus assay with urine derived cells (UDC): A review of its application in human studies investigating genotoxin exposure and bladder cancer risk |
|
| Armen Nersesyan,Michael Kundi,Michael Fenech,Claudia Bolognesi,Miroslav Misik,Georg Wultsch,Michaele Hartmann,Siegfried Knasmueller | | Mutation Research/Reviews in Mutation Research. 2014; | | [Pubmed] | [DOI] | | | 2 |
DNA and Oxidative Damages Decrease After Ingestion of Folic Acid in Patients with Type 2 Diabetes |
|
| Lazalde-Ramos, B.P. and Zamora-Perez, A.L. and Sosa-Mac├şas, M. and Guerrero-Vel├ízquez, C. and Z├║├▒iga-Gonz├ílez, G.M. | | Archives of Medical Research. 2012; 43(6): 476-481 | | [Pubmed] | | | 3 |
DNA and Oxidative Damages Decrease After Ingestion of Folic Acid in Patients with Type 2 Diabetes |
|
| Blanca Patricia Lazalde-Ramos,Ana Lourdes Zamora-Perez,Martha Sosa-MacÝas,Celia Guerrero-Velßzquez,Guillermo Moises Z˙˝iga-Gonzßlez | | Archives of Medical Research. 2012; 43(6): 476 | | [Pubmed] | [DOI] | |
|
 |
|






