|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2012 | Volume
: 18
| Issue : 2 | Page : 187-192 |
| |
Genetic and environmental determinants of menstrual characteristics
Shayesteh Jahanfar
Department of Epidemiology and Biostatistics, School of Population and Public Health, University of British Columbia,
| Date of Web Publication | 8-Sep-2012 |
Correspondence Address:
Shayesteh Jahanfar
School of Population and Public Health, University of British Columbia, 2206 East Mall, Vancouver BC V6T 1Z3, Canada
 Source of Support: None, Conflict of Interest: None  | 2 |
DOI: 10.4103/0971-6866.100759

 Abstract Abstract | | |
Background: The impact of women's menstrual cycle on her quality of life, health, work, and community is substantial. Menstrual disturbance is linked with general ill conditions such as migraine, asthma, and endocrinopathies. The clinical significance of medical interventions to prevent these conditions becomes clear if the role of genetic or environment is clarified.
Aims: To identify the genetic and environmental contribution on menstrual characteristics.
Setting and Design: This was a cross-sectional study in 2 Asian countries.
Materials and Methods: 2 cohorts of monozygotic and dizygotic twins born between (1945-1988, n = 122) and (1951-1993, n = 71) were taken. A standard questionnaire was designed inclusive of socio- demographic characteristics of subjects as well as menstrual history (duration, interval, amount, irregularity). Subjects were interviewed by phone.
Statistical Analysis: Quantitative variables were analyzed using Falconars' formula as well as maximum likelihood analysis. Structural modeling was then applied to twin correlations to provide estimates of the relative genetic and/or environmental factors contribution in determining the measured trait.
Results: Menstrual characteristics were found to be under environmental influence where the best fitting model for menstrual interval and duration was common environment. CDF plotting confirmed the results for both variables. Proband-wise concordance analysis for amount of menstruation, amenorrhea, and irregular menstruation revealed no genetic influence. The best fitting model for menstrual irregularity was CE (C73%, E27%). The same model was defined for amenorrhea (C48%, E52%).
Conclusions: Environmental factors are most likely responsible to determine the menstrual flow, its integrity, and regularity. These factors need to be studied further.
Keywords: Environment, genetic, menstrual cycle, twins
How to cite this article:
Jahanfar S. Genetic and environmental determinants of menstrual characteristics. Indian J Hum Genet 2012;18:187-92 |
 Introduction Introduction | |  |
Menstrual cycles act as evident indicators of underlying reproductive health. [1] Menstrual dysfunction reveals both infertility and increases future risk of various chronic diseases such as diabetes, breast cancer, cardiovascular diseases. [2],[3],[4],[5] Dysfunctional menstrual cycles can begin from adolescence and persist for many years and throughout reproductive life-span causing physical, psychological, and economical strains on women's life. [6] Menstrual dysfunction can be assessed via biochemical assays or menstrual history. While biochemical assays have added advantage of measuring hormonal levels and the potential to estimate the ovulation time, menstrual cycle characteristics are easy to observe, cost-effective and conveniently monitored by women themselves. Menstrual history is inclusive of cycle length, bleeding length, amount of bleeding, and regularity of cycles. Menstrual length has been used as a non-invasive clinical marker of ovarian steroid production. [7] Regularity of cycles is a marker of smooth function of hypothalamus pituitary ovarian axis.
Genetic influences have been reported on menstrual characteristics. [8],[9],[10],[11],[12],[13] However, methodological problems such as small samples [14] and inability to distinguish between proportion of genetic and environmental influences clouds the conclusions. Moreover, the environmental influences have been studied on menstrual patterns. [15] The question remains whether menstrual characteristics are under genetic or environmental factors. We investigated genetic and environmental variation of menstrual characteristics among identical and non-identical twins.
 Materials and Methods Materials and Methods | |  |
Grant was provided by Royal College of Medicine, Perak; University of Kuala Lumpur. An ethical approval was obtained from National Malaysian Research Registry and Iran Avecina Research Center. Participants were members of 2 national twin registries of Iran and Malaysia. 2 cohorts (born between 1945-1988, n = 122) and (born between 1951-1993, n = 71) were taken as volunteers. Homogeneity testing was performed to ensure the ability to collapse data from 2 nations. Twin recruitment and details related to the registries can be found elsewhere. [16],[17] A standard questionnaire was designed inclusive of socio-demographic characteristics of subjects as well as menstrual history. 3 items concerning similarity in appearance and being mistaken by others were used to determine zygosity. [18] Such questionnaires have been shown to give at least 95% agreement with diagnosis based on extensive blood-typing. [19] Menstrual history included duration (defined as number of bleed days), interval (defined as from the first day of last menstrual period till the first day of next period in day), and amount of menstruation clarified by the number of soaked pads used per day and categorized as normal or abnormal; where normal defined as having a flow of blood or changing 2 to 3 pads per day during menstruation on average; while abnormal defined as either spotting or excessive bleeding. Amenorrhea was defined as having 0-2 cycle per year, and finally irregular menstrual bleeding was recorded as a qualitative variable where subjects replied, yes or no when asked: "Have you had regular menstrual cycle?" All of the above questions were pertained to events happening during last year.
Statistical analysis: Quantitative variables such as interval and duration of menstrual flow were analyzed using Falconars' formula (1996) as well as maximum likelihood analysis. [20] Falconer's formula for estimation of heritability (h 2 ) is h 2 = 2 (rMZ - rDZ ), where r stand for correlations between twin 1 and 2. Shared environment is estimated by MZ correlation deducted from heritability value C = (r MZ - h 2 ). Non- shared environment or E 2 is a reflection of the degree to which identical twins raised together are dissimilar, E = (1- r MZ ).
Use of plots to visualize data is highly recommended in every twin analysis. [21] The plot is constructed by first computing the absolute within-pair differences for twin data, d ik = /Y i1K , Y i2K /, where the data consist of n1 pairs Y iK = (Y i1K , Y i2K ), K = 1,…, n1, where i stands for zygosity. Then, the differences are ranked separately for each zygosity and so called "Cumulative Distribution Function" (CDF); Fi (X) = (numbers of d ik values X)/n i are constructed for each zygosity 1. These 2 functions are then overlaid to form plots. Since our sample size was different for DZ and MZ twins, comparison were made using corresponding percentile values of absolute within-pair differences. Interpretations of the plots are based on the following points. [21] If a genetic effect exists, then typically many of the higher percentiles DZ differences would exceed the corresponding MZ differences as the DZ pairs are less similar for the trait. If the traits have no genetic influence; then the DZ differences appear approximately at the same level of corresponding MZ absolute differences.
Moreover, using the plots is beneficial in finding outliers that can distort the results of the twin data analysis and may not be possible to detect on univariate plots of the raw data.
Then, a more comprehensive genetic analysis was conducted via maximum likelihood analysis using MX software. Univariate twin model was applied [19] to the variance and covariance matrices derived from MZ and DZ twin data. The full model included additive genetic (A), shared environment (C), and non-shared environmental variances (E). MX compares Model 1 (Y = a 2 +c 2 +e 2 ) and model 2 (Y = a 2 +e 2 ) investigating the effect of common environment. Another comparison also took place (Model 1 and 3 where Y = a 2 +c 2 ) to investigate the role of not-shard environment. Finally, the best fit model was introduced, and the proportion of genetic factor or environmental factor was given in percentage.
Qualitative variables such as amount of menstrual flow, however, were tested for genetic heritability via probandwise correlation. Contingency tables were constructed for absence or presence of each condition (abnormal bleeding, irregular menstruation, amenorrhea) in both MZ and DZ twin pairs. If probandwise correlation is higher among MZ than that of the DZ, a heritable effect is indicated. This is derived from 2d/(2d+b+c); where d is the number of discordant twins, and b, c is the number of concordant twins for the measured trait.
Genetic modeling for qualitative variables was based on assumption that there is a threshold of liability dividing subjects into 2 categories of affected and unaffected. Correlation in liability among twins was estimated from the frequencies of concordant and discordant pairs (tetrachoric correlation). Structural modeling was then applied to the MZ and DZ twins correlations to provide estimates of the relative genetic and/or environmental factors contribution in determining the trait. The principal models used are similar to that of quantitative variables inclusive of additive component (A) as well as environmental variance inclusive of common or shared environment (C) and unique or non-shared environment (E). For MZ twins, correlations between additive genetic factors between the twin 1 and twin 2 is 1. For DZ twins, these values are 0.5. Correlations are unity between common environmental factors and zero between unique environmental factors.
 Result Result | |  |
Quantitative Variables
The mean for menstrual duration was 5.81 ± 1.28 with minimum of 2 days and maximum of 10 days. Mean value for the interval of menstruation was 29.75 ± 9.41 with minimum of 22 and maximum of 40 days.
Falcouner's formula [h 2 = 2 (r MZ - r DZ )] showed a small contribution for genetic factors for menstrual duration (h 2 = 0.17). Modern genetic analysis confirmed these result as the heritability was found to be only 2% [Table 1]. The best fitting model for both menstrual interval and duration follows a pattern of environmental effect. | Table 1: Univariate twin analysis for reproductive events inclusive of genetic (A), common environment (C) and shared environment (E) effect and best fitting model estimation
Click here to view |
Use of plots to visualize data is highly recommended in every twin analysis. [21] Quantitative variables were examined using CDF plots. Since our groups of MZ and DZ twins were different in sample sizes, comparison was made using corresponding percentile values of absolute within-pair differences. For duration and interval of menstrual cycle [Figure 1] and [Figure 2], 2 lines pertaining to MZ and DZ overlapped for a long while before departure from each other for more disparate data, hence the effect of environment.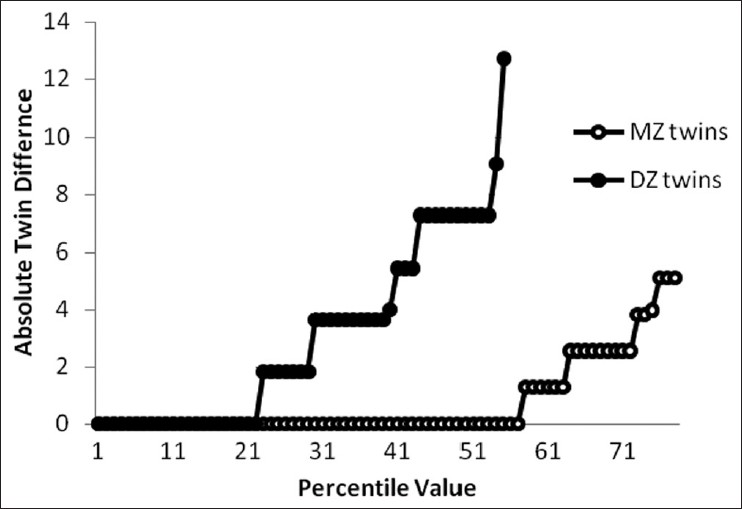 | Figure 1: Cumulative distribution function plot of menstrual duration (day)
Click here to view |
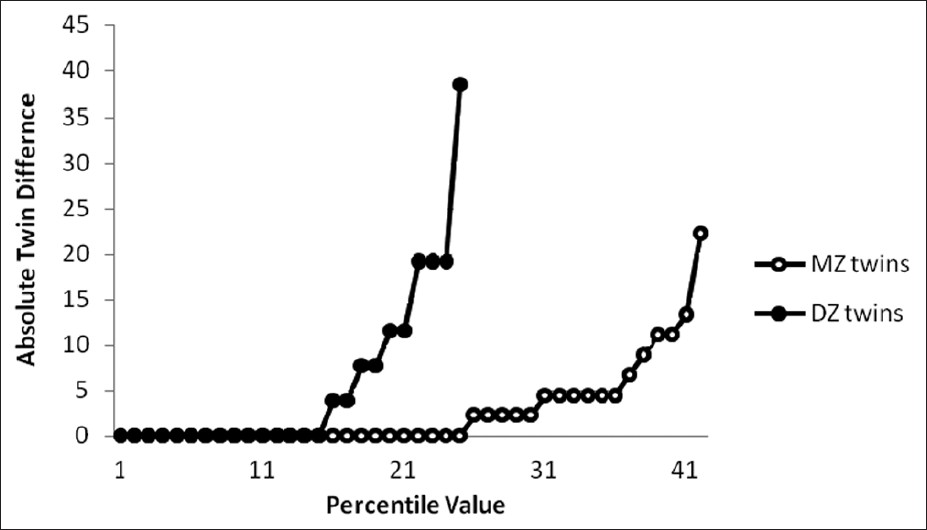 | Figure 2: Cumulative distribution function plot of menstrual interval (day)
Click here to view |
Qualitative Variables
Probandwise concordance rate for MZ and DZ twins was calculated for 2 measured variables inclusive of irregular menstruation, and abnormal bleeding. The MZ:DZ ratio was 1.00 for irregular menstruation and 0.692 for abnormal bleeding. This ratio should be higher than 1 to suggest genetic influence. In a twin sample, correlation in liability among twins can be estimated from the frequencies of concordant and discordant pairs, and it is called tetrachoric correlation. The assumption behind this correlation is that the underlying liability is following a normal distribution or can be transformed to one. Tetrachoric correlations for MZ and DZ for both the above-mentioned variables were below 1 as shown in [Table 2].
Structural equation modeling was then applied to the MZ and DZ twins correlations to provide estimates of the relative genetic and/or environmental factors contribution in determining the trait. For each phenotype, there are genetic variances inclusive of additive component (A) and a dominant component (D) as well as environmental variance inclusive of common or shared environment (C) and unique or non-shared environment (E). [Table 3] shows the best fitting model for 2 qualitative variables to be under environmental factors.
 Discussion Discussion | |  |
The impact of women's menstrual cycle on her quality of life, health, work, and community is substantial. [15] Menstrual disturbance is linked with general ill conditions such as migraine, asthma, [22],[23] and endocrinopathies such as hypothyroidism, asthma immune system. [24],[25] In other words, a woman's menstrual pattern is an important indicator of her health.
Characteristics of menstrual cycle are known by its length, duration, amount, and regularity. Length is defined as the beginning of menstrual flow of each month to the first day of menstrual flow of the next month. Length of menstrual cycle ranges between 21 to 41 days, and anything above or below this range is considered abnormal. [22] A study found that the mean total length of menstrual cycle for 141 healthy women was 28.9 days (SD = 3.4) with 95% of the cycles between 22 and 36 days. [7] According to this study, menstrual length less than 21 days is called polymenorrhea and above 42 days is entitled as oligomenorrhea.
Duration is known as the period of menstrual flow. The average menstrual duration is known to be about 7 days, and the range could be between 3 - 10 days.
Predictive value of menstrual cycle pattern is important when counseling women with irregular menstrual cycles or oligomenorrhea. Subjects with endocrine disturbance may be at risk of developing ovarian dysfunction and other endocrinopathies such as polycystic ovary syndrome or already are suffering from it. [16]
Many environmental factors may affect characteristics of menstrual cycle including workplace, [26] caffeine consumption, [27] smoking, [28] occupation, [29] physical activity, [30] diet, [31] age, [32] weight, [33] exposure to organic solvent, [34] medical conditions, and lifestyle factors. [35] Intra-uterine effect of diethylstilbestrol on menstrual length is also reported. [36] Our study result is suggesting that environmental factors, unique to the individual, play a major role in determining the characteristics of menstrual flow inclusive of duration and interval. The heritability value for these 2 characteristics were less than 0.1% for interval and were about 2% for duration while model fitting analysis suggested 95% of non-shared environmental effect for interval and 67% of shared environmental effect for duration. Moreover, CDF plot of these 2 variables show overlap of lines for difference percentile of DZ and MZ twins. Other menstrual characteristics studied were inclusive of irregular menstruation and abnormal menstrual amount, both of which were found to be under environmental effect. Probandwise concordance rate (MZ: DZ) for these variables were less than 2, and the best fitting model was CE.
Our result was different than others. Menstrual and premenstrual symptoms have been studied in an American cross-sectional genetic design, and the heritability estimate of 0.34, 0.41, and 0.40 has been reported for flow, pain, and menstrual limitation, respectively. [18] The corresponding numbers for these variables in an older Australian study of female twins were 22, 38, and 36%, respectively. [10] The first longitudinal study about menstrual disorders on twins was done by Treloar [13] on 728 pairs (466 MZ and 262 DZ). Genetic factors accounted for 39% of the longitudinally stable variation in menstrual flow, 55% for pain, and 77% for limitation. The remaining stable variance was pertained to non-shared environmental factors (61, 45, and 23%, respectively). The author concludes that during 8 years of follow-up, the stable variance was largely environmentally influenced for menstrual flow, was partly determined by genetic and partly by non-shared environmental factors in the case of pain, and was due almost entirely to genetic influences for limitation by periods. These result showed that the same genetic influences are operating throughout the reproductive life span.
 Acknowledgement Acknowledgement | |  |
This study was done using financial support of University Kuala Lumpur. I would like to thank twins who participated in the study and research assistance who collected data. Special thanks goes to Mrs. Haleh Maleki for her coordination and Dr. Sadeghi for his dedicated collaboration. I like to thank Prof. Lye Mann Sunn and Dr. Isthrinayagy A/P S. Krishnarajah for their advice and comments.
 References References | |  |
| 1. | Popat VB, Prodanov T, Calis KA, Nelson LM. The menstrual cycle a biolosgical marker of general health in adolescents. Ann N Y Acad Sci 2008;1135:43-51. 
[PUBMED] |
| 2. | Krassas GE. Thyroid disease and female reproduction. Fertil Steril 2000;74:1063-70. 
[PUBMED] |
| 3. | Yeshaya A, Orvieto R, Dicker D, Karp M, Ben-Rafael Z. Menstrual characteristics of women suffering from insulin-dependent mellitus. Int J Fertil Menopausal Stud 1995:40:269-73. 
[PUBMED] |
| 4. | Livshits A, Sediman DS. Fertility issues in women with diabetes. Womens Health (Lond Engl) 2009;5:701-7. 
|
| 5. | Bertuccio P, Tavani A, Gallus S, Negri E, La Vecchia C. Menstrual and reproductive factors and risk of non-fatal acute myocardial infarction in Italy. Eur J Obstet Gynecol Reprod Biol 2007;134:67-72. 
[PUBMED] |
| 6. | Deligeoroglou E, Tsimaris P, Deliveliotou A, Christopoulos P, Creatsas G. Menstrual disorders during adolescence. Pediatr Endocrinol Rev 2006;3 Suppl 1:150-9. 
[PUBMED] |
| 7. | Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs 2006;35:376-84. 
[PUBMED] |
| 8. | Kantero RL, Widholm O. Correlations of menstrual traits between adolescent girls and their mothers. Acta Obstet Gynecol Scand (Suppl) 1971;14:30-6. 
|
| 9. | van den Akker OB, Stein GS, Neale MC, Murray RM. Genetic and environmental variation in menstrual cycle: Histories of two British twin samples. Acta Genet Med Gemellol (Roma) 1987;36:541-8. 
[PUBMED] |
| 10. | Silberg JL, Martin NG, Heath AC. Genetic and environmental factors in primary dysmenorrheal and its relationship to anxiety depression and neuroticism. Behav Genet 1987;17:363-83. 
[PUBMED] |
| 11. | Kendler SK, Karkowski LM, Corey LA, Neale MC. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry 1988;155:1234-40. 
|
| 12. | Kendler KS, Silberg JL, Neale MC, Kessler RC, Heath AC, Eaves LJ. Genetic and environmental factors in the etiology of menstrual, premenstrual and neurotic symptoms: A population-based twin study. Psychol Med 1992;22:85- 100. 
|
| 13. | Treloar SA, martin NG. Age at menarche as a fitness triat: Nonadditive genetic variance detected in a large twin sample. Am J Hum Genet 1990;47:137-48. 
[PUBMED] |
| 14. | Dalton K, Dalton ME, Guthrie K. Incidence of the premenstrual syndrome in twins. Br Med J (Clin Res Ed) 1987;295:1027-8. 
[PUBMED] |
| 15. | Treloar SA, Heath AC, Martin NG. Genetic and environmental influences on premenstrual symptoms in an Australian twin sample. Psychol Med 2000;32:25-38. 
|
| 16. | Jahanfar S, Maleki H, Mosavi AR. Leptin and its association with polycystic ovary syndrome- A twin study. Gynecol Endocrinol 2004;18:327-34. 
[PUBMED] |
| 17. | Jahanfar S, Maleki H, Mosavi AR. Subclinical eating disorder, polycystic ovary syndrome-Is there any connection between these two conditions through leptin - A twin study. Med J Malaysia 2005;60:441-6. 
[PUBMED] |
| 18. | Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves L. A test of equal-environment assumption in twin studies of psychiatric illness. Behav Genet 1993;23:21-7. 
|
| 19. | Martin NG, Martin PG. The inheritance of scholastric abilities in a sample of twins. I. Ascertainments of the sample and diagnosis of zygosity. Ann Hum Genet 1975;39:213-8. 
|
| 20. | Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Chapter 1, Model fitting functions and optimization. Dordrecht, The Nethrelands: Kuluwer Academic; 1992. 
|
| 21. | Williams CJ, Christian JC. Plots for examination of univarite twin data. Comput Biomed Res 1992;25:527-37. 
[PUBMED] |
| 22. | Newman LC, Lipton RB, Lay CL, Solomon S. A pilot study of oral sumatriptan as intermittent prophylaxis of menstruation-related migraine. Neurology 1998;51:307-9. 
[PUBMED] |
| 23. | Martinez-Moragon E, Plaza V, Serrano J, Picado C, Galdiz JB, Lopez-Vina A, et al. Near-fetal asthma related to menstruation. J Allergy Clin Immunol 2004;113:242-4. 
|
| 24. | Krassas GE, Pontikides N, Kaltsas TH, Papadopoulou PH, Paunkovic J, Paunkovic N, et al. Disturbancea of menstruation in hypothyroidism. Clinical Endocrinol (Oxf) 1999;50:655-9. 
|
| 25. | Critchley HO, Kelly RW, Brenner RM, Bairs DT. The endocrinology of menstruation-a role for the immune system. Clin Endocrinol (Oxf) 2001;55:701-10. 
|
| 26. | Fenster L, Quale C, Waller K. Caffeine consumption and menstrual function. Am J Epidemiol 1999;149:550-7. 
|
| 27. | Fenster L, Waller K, XChen J. Psychological assessment of fecundability of female semiconductor workers. Am J Ind Med 1999;28:817-31. 
|
| 28. | Windham GC, Elkin EP, Swan SH. Cigarette smoking and effects on menstrual function. Obstet Gynecol 1999;93:59- 65. 
|
| 29. | Gold EB, Ezkenazi B, Hammond SK. Prospectively assessed menstrual cycle characteristics in female waferfabrication and nonfabrication semiconductor employees. Am J Ind Med 1995;28:799-815. 
|
| 30. | Strenfeld B, Jacobs MK, Quesenberry CP, Gold EB, Sowers MF. Physical activity and menstrual cycle characteristics in two prospective cohort. Am J Epidemiol 2002;156:402-9. 
|
| 31. | Bagga D, Ashley JM, Geffrey SP. Effects of a very low fat, high fiber diet on serum hormones and menstrual function. Implications for breast cancer prevention. Cancer 1995;76:2491-6. 
|
| 32. | Dennerstein L, Gotts G, Brown JB. Effects of age and non-hormonal contraception on menstrual characteristics. Gynecol Endocrinol 1997;11:127-33. 
[PUBMED] |
| 33. | Harlow SD, Matanoski GM. The association between weight, physical activity, and stress and variation in the length of the menstrual cycle. Am J Epidemiol 1991;133:38- 49. 
[PUBMED] |
| 34. | Cho SI, Damokosh AI, Ryan LM, Chen D, Hu YA, Smith TJ, et al. Effects of exposure to organic solvents on menstrual cycle length. J Occup Environ Med 2001;43:567-75. 
[PUBMED] |
| 35. | Roeland AS, Baird DD, Long S, Wegienka G, Harlow SD, Alavanja M, et al. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology 2002;13:668-74. 
|
| 36. | Hornsby PP, Wilcox AJ, Weinberg CR, Herbst AL. Effects on the menstrual cycle of the in utero exposure to diethylstilbestrol. Am J Obstet Gynecol 1994;170:709-15. 
[PUBMED] |
[Figure 1], [Figure 2]
[Table 1], [Table 2], [Table 3]
| This article has been cited by | | 1 |
Does midwifery philosophy affect perceptions of students regarding female reproduction? |
|
| Anita Jug Došler,Ana Polona Mivšek | | Health Sociology Review. 2015; : 1 | | [Pubmed] | [DOI] | | | 2 |
Chronobiology awareness concerning the menstrual cycle |
|
| Shilpa Shah,Gerhard Meisenberg | | Biological Rhythm Research. 2014; : 1 | | [Pubmed] | [DOI] | | | 3 |
Malaysian twin registry |
|
| Jahanfar, S. and Jaffar, S.H. | | Twin Research and Human Genetics. 2013; 16(1): 246-247 | | [Pubmed] | |
|
 |
|






