|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2012 | Volume
: 18
| Issue : 3 | Page : 326-331 |
| |
Pharmacogenetic typing for oral anti-coagulant response among factor V Leiden mutation carriers
Risha Nahar1, Renu Saxena2, Roumi Deb3, Ishwar C Verma2
1 Centre of Medical Genetics, Sir Ganga Ram Hospital, Rajinder Nagar, New Delhi; Amity Institute of Biotechnology, Amity University, Uttar Pradesh, India
2 Centre of Medical Genetics, Sir Ganga Ram Hospital, Rajinder Nagar, New Delhi, India
3 Amity Institute of Biotechnology, Amity University, Uttar Pradesh, India
| Date of Web Publication | 4-Mar-2013 |
Correspondence Address:
Ishwar C Verma
Director, Center of Medical Genetics, Sir Ganga Ram Hospital, Rajinder Nagar - 110 060, New Delhi
India
 Source of Support: The research fund for the above work was granted by Sir Ganga Ram Hospital, New Delhi, India, Conflict of Interest: None  | 2 |
DOI: 10.4103/0971-6866.107987

 Abstract Abstract | | |
Context: Factor V Leiden mutation is the most common inherited predisposition for hypercoagulability and thereby a common genetic cause for initiation of oral anti-coagulation therapy. There is a dearth of knowledge of coumarin response profile in such thrombophilic population.
Aims: The current pilot study aims to estimate coumarin sensitivity in an Indian cohort with an inherited thrombophilia risk factor (Factor V Leiden mutation carriers) based on the observed frequency of CYP2C9 *2, *3 and VKORC1-1639G >A genotype combinations.
Settings and Design: A retrospective study carried out in a tertiary health care center in India.
Materials and Methods: Carriers of FVL mutation were genotyped for CYP2C9 (*2, *3) and VKORC1 (-1639G >A) variants by PCR-RFLP technique.
Statistical Analysis Used: Chi-square test to analyze difference in expected and observed genotype frequency.
Results: Sixty-one (n = 61) unrelated carriers of FVL mutation were observed in the 13 years study period. The allele frequency of CYP2C9 *2, CYP2C9 *3, and VKORC1-1639A in this cohort was 0.06, 0.11, and 0.16, respectively. Six (9.7%) individuals had two of the three variant alleles (heterozygous or homozygous), and 28 (45.9%) were heterozygous for at least one polymorphism.
Conclusions: Pre-prescription genotyping for coumarin drugs, if introduced in Indians with inherited thrombophilia (in whom oral anti-coagulant therapy may be necessary), is likely to identify 9.7% (hypersensitive) subjects in whom the optimum anti-coagulation may be achieved with reduced dosages, 44.3% (normal sensitivity) who may require higher dose and also 55.6% (hyper and moderate sensitivity) subjects who are likely to experience bleeding episodes.
Keywords: Acenocoumarol, bleeding, factor V Leiden, India, oral anti-coagulant, pharmacogenetic, prothrombin, thrombosis, thrombophilia, warfarin
How to cite this article:
Nahar R, Saxena R, Deb R, Verma IC. Pharmacogenetic typing for oral anti-coagulant response among factor V Leiden mutation carriers. Indian J Hum Genet 2012;18:326-31 |
How to cite this URL:
Nahar R, Saxena R, Deb R, Verma IC. Pharmacogenetic typing for oral anti-coagulant response among factor V Leiden mutation carriers. Indian J Hum Genet [serial online] 2012 [cited 2016 Jun 1];18:326-31. Available from: http://www.ijhg.com/text.asp?2012/18/3/326/107987 |
 Introduction Introduction | |  |
Thrombosis is one of the leading causes of death worldwide. [1] In India, the incidence of venous thromboembolism is reported to be 17.5 per 10,000 hospital admissions. Risk factors (surgery, malignancy, obesity, increased age) for venous thromboembolism has been reported in 54% of hospitalized patients. [2] Risk of venous thromboembolism in an individual is influenced by the combination of his or her baseline predisposition for thrombosis (deficiency of natural clotting inhibitors or elevated procoagulants or increased fibrinolytic factors) and the magnitude of the acquired risk factors (pregnancy, smoking, injury, increasing age, obesity, surgery, immobility). [3] Amongst these, activated protein C resistance is the most common trigger and is seen in 20-50% patients with inherited thrombophilia. Factor V Leiden (FVL) mutation (1691G >A; R506Q) accounts for 92% of cases with activated protein C resistance, [4] making it the most common genetic risk factor for thromboembolism worldwide. [5],[6] Heterozygous and homozygous carriers of the mutation have a seven-fold and 80-fold increase in thrombosis risk, respectively. [3] This maybe further augmented by pregnancy (9-fold), use of oral contraceptives (36-fold), and hormone replacement therapy (13- to 16-fold). [4] Carriers of FVL are also known to have an earlier age of onset of thrombosis as compared to non-carriers. [7]
Studies have indicated the use of prophylactic anti-coagulation therapy in carriers of FVL during high-risk situations known to provoke thrombosis such as hospitalization for surgery or other medical reasons. [8],[9] Latest Guidelines by the American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel [10] suggest six months of post-partum prophylactic anti-coagulation therapy (Vitamin K antagonists with target INR 2-3) for pregnant women known to be homozygous for FVL or Prothrombin 20210A mutation irrespective of their history of venous thromboembolism.
Warfarin and Acenocoumarol are the most widely prescribed anti-coagulant for the treatment and prevention of venous and arterial thromboembolic events. Although very effective, usage of these drugs is limited by the difficulty in optimizing therapeutic dosage and fear of inducing bleeding or severe hemorrhage. Prospective studies carried out in tertiary health care centers in India [11],[12] and the world [13] revealed that 5% to 6.9% of all hospital admissions were due to adverse drug reactions, of which warfarin-induced bleeding was ranked as one of the most common causes. Both genetic and clinical factors are known to contribute towards inter-individual variation to warfarin. In year 2010, the Food and Drug Administration, U.S.A., approved revised prescribing information on warfarin (Coumadin), stressing on the effect of the three polymorphisms (CYP2C9 * 2, *3 and VKORC1-1639G >A) on dose and bleeding with specific recommendations of dose ranges for individuals with various genotype combinations. [14]
CYP2C9 is the principal enzyme involved in the metabolic clearance of the more potent S-enantiomer of warfarin and is responsible for a large inter-individual variation in dose. Studies indicate that patients with the common, functionally defective *2 (430C0 >T) and *3 (1075A >C) allelic variants of the Cytochrome P450 enzyme 2C9 (CYP2C9) require significantly lower doses, take longer time to achieve stabilized dose, and are at a higher risk for serious bleeding that are patients without these variants. [15],[16] Coumarin-based anti-coagulants act by inhibition of vitamin K epoxide reductase complex 1 (VKORC1), leading to depletion of reduced vitamin K and thereby interfering with the synthesis of vitamin K-dependent clotting factors, resulting in anti-coagulation. Studies have revealed a non-coding polymorphism in the VKORC1 (-1639G >A) to be associated with warfarin and acenocoumarol dose variability. [17],[18],[19]
There is a dearth of knowledge of coumarin response profile in the Asian-Indian population, as no pharmacogenetic studies have been carried out with respect to coumarin anti-coagulants in the population till date. Hence, the current preliminary study was undertaken with an aim to predict coumarin sensitivity in an Asian-Indian cohort with an inherited thrombophilia risk factor (Factor V Leiden mutation carriers). This specific cohort was chosen, as they may have a higher possibility of requiring oral anti-coagulation therapy than the general population. Based on the frequency of the CYP2C9 *2, *3 and VKORC1-1639G >A genotype combinations, the study aims to identify the proportion of those who are (i) hypersensitive to coumarin, as they may require reductions in coumarin dose and have an increased risk of bleeding and, (ii) normal sensitivity to coumarin, as they are known to require higher dose to achieve therapeutic anticoagulation.
 Materials and Methods Materials and Methods | |  |
The study was carried out at the Centre of Medical Genetics in a tertiary health care facility in India. The study protocol was approved by the Hospital's Research Ethics Committee and is in accordance with the ethical standards of the World Medical Association's Helsinki Declaration.
Study population
Retrospective analysis of Factor V Leiden mutation data in the 13 years period from 1997 through 2010 was carried out. Reports of individuals referred for FVL mutation molecular genetic testing at the study Center were analyzed, and all those detected as carriers of at least one mutant allele were included in the current study. Written informed consent was obtained from all subjects prior to the test. These samples were referred for testing by physicians across the country from departments of vascular surgery, obstetrics, and medical genetics.
Detection of factor V Leiden mutation (1691G >A)
DNA was isolated from whole blood using standard salt-extraction method. [20] Genotyping of F5 1691G >A variant was done by restriction enzyme digestion of PCR-amplified DNA based on previously published protocol [21] with modifications.
Genotyping of CYP2C9 *2 (c.430C >T; rs1799853); CYP2C9 *3 (c.1075A >C; rs1057910); VKORC1 *2 (c.-1639G >A; rs9923231)
The three polymorphisms were genotyped using polymerase chain reaction followed by restriction enzyme digest as described previously [22],[23] with modifications.
Statistical analysis
Chi-square test was applied (using SPSS statistical package version 15.0) to analyze if the genotype frequencies were in Hardy Weinberg equilibrium. A P value of less than 0.05 was considered to be statistically significant.
 Results Results | |  |
In the 13 year period from 1997 through 2010, out of the 1368 individuals tested, 61 unrelated individuals (18 males; 43 females) were detected to be carriers of the FVL mutation (two homozygous and 59 heterozygous) by molecular genetic testing at the study Center. The common reasons for referral were pregnancy-associated complications, venous or arterial thrombosis, and activated protein C resistance. The age of the subjects ranged from 24 to 52 years (mean = 31 + 7.3 years), and majority (59, 96.7%) were ethno-geographically North Indians (Indo-European linguistic ethnic group). The other two belonged to West India origin.
The observed genotype and allele frequencies of CYP2C9 (*2, *3) and VKORC1-1639G >A [Table 1] was in Hardy Weinberg Equilibrium. Analysis showed that 32.8% (20) were carriers of the CYP2C9 * 2 or *3 alleles, and 29.5% (18) were carriers of the VKORC1-1639A allele. The combined CYP2C9/VKORC1 genotype profile [Figure 1] reveals that six (9.7%) individuals had two of the three variant alleles (heterozygous or homozygous for CYP2C9 * 2, *3, VKORC1-1639G > A). Similar fractions of study population were observed to be heterozygous for a variant allele (28, 45.9%) and homozygous for wild-type alleles for all three polymorphisms (27, 44.3%).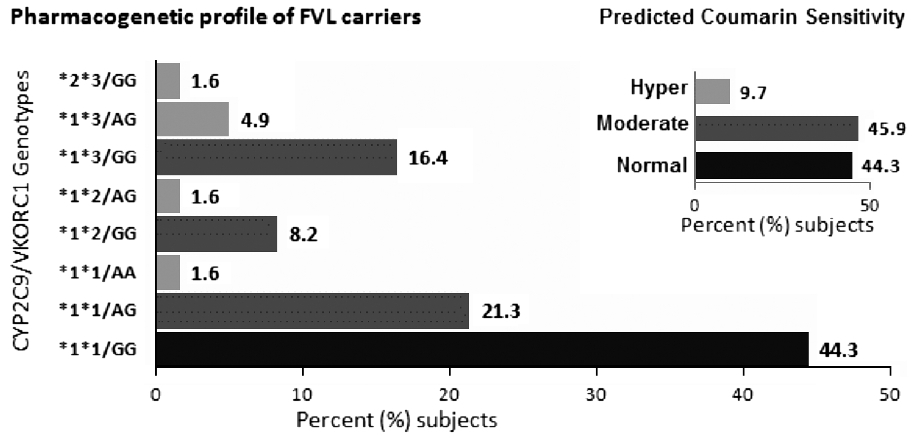 | Figure 1: CYP2C9/VKORC1 genotype profile and (inset) frequency of coumarin-response genotype groups in subjects at high risk for thrombophilia (FVL carriers): Individuals with two variant CYP2C9/VKORC1 genotypes (either compound heterozygous or homozygous) were grouped as 'hyper' sensitive (included *2 * 3/GG; *1 * 3/AG, *1 * 2/AG and *1 *1/AA) and are indicated by grey bars. Those with single heterozygous polymorphism including *1 * 1/AG; *1 *2/GG, and *1 * 3/GG were grouped to have 'moderate' sensitivity and are indicated with dark grey bars. The wild-type CYP2C9/VKORC1 (*1 *1/GG) are indicated with black bars and comprise those with 'normal' sensitivity. The inset bar graph depicts the total frequency of the three estimated coumarin sensitivity groups
Click here to view |
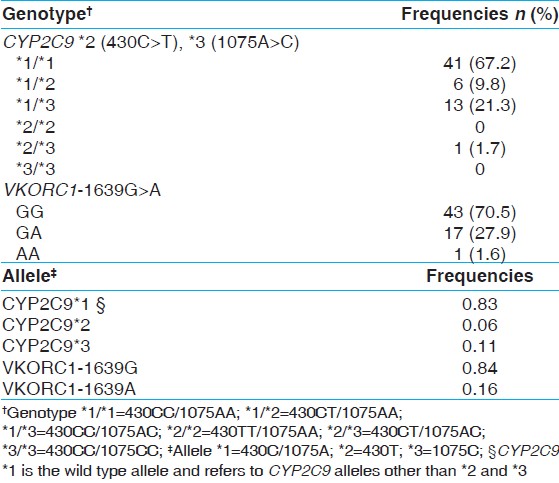 | Table 1: Genotype and allele frequencies of CYP2C9 and VKORC1 in factor V Leiden mutation carriers
Click here to view |
 Conclusion Conclusion | |  |
Previous studies have proved that despite individualization of dose to compensate for variation in patients' age, weight, diet, clinical indication, and concurrent use of other medications, the three common genetic variants (CYP2C9 *2, *3 and VKORC1-1639G >A) have a significant effect on a variety of anti-coagulation-related outcomes such as therapeutic dose requirement, time taken to achieve stable dosing, and adverse effects. [23],[24] The VKORC1 polymorphism -1639A accounts for 19% to 30% of variance in the dose of warfarin, while CYP2C9 SNPs (*2, *3) have a contribution of 3.2% to 12%. [25],[26] With regard to dose variability of acenocoumarol, CYP2C9 * 3, VKORC1-1639A and age are known to explain 50% to 55% inter-individual variance. [27],[28]
The present study analyzes the frequency of these common CYP2C9 and VKORC1 SNPs in a total of 122 chromosomes (n = 61) of individuals at high risk for hereditary thrombophilia (FVL mutation carriers). The study shows that 55.6% of the study population with one or more variant genotypes (CYP2C9 * 2, *3, VKORC1-1639G >A) are likely to be sensitive to coumarin anti-coagulants and may require less than average dosages and harbor an increased risk for drug-induced adverse effects. Similarly, a smaller study in a multiethnic population [29] reported that 83% of individuals at high risk of thrombophilia (FVL and prothrombin mutation carriers) were carriers of the same three polymorphisms. This magnified proportion of warfarin-sensitive group in the previous study [29] may be attributed to variation in ethnic origin and/or sample size ( n = 35 versus n = 61 in present study).
FVL mutation contributes to 15.8% of venous thrombosis in Asian Indians [30] and 31.8% in Caucasians. [31],[32],[33] Although the thrombophilic prothrombin mutation 20210G >A (F2) is common in the white and Caucasian populations, it is rare in the Asian Indian general population. [30],[34],[35] This was confirmed by the findings at the Center of Medical Genetics, Sir Ganga Ram Hospital, India. In the 13-year period (1997 through 2010), none of the cases referred for prothrombin mutation analysis 20210G >A were positive for the mutation. Considering the low frequency of the mutation in the Asian Indians, the present study did not include any patients with the prothrombin mutation.
The current pilot study was carried out in a cohort of FVL mutation carriers who were not on prophylactic or therapeutic oral anti-coagulant therapy, hence accurate maintenance dose estimation and prevalence of bleeding events was not viable in the present cohort. Additionally, lack of data on non-genetic determinants of coumarin response (co-morbidities, concomitant interacting medications, weight, height, smoking status, and vitamin K intake) may reduce the efficacy of predicted coumarin sensitivity profiles in the current study. However, the study does take into account the three most important genetic markers for coumarin sensitivity, which have also been approved for estimating dose by the U.S.A. Food and Drug Administration.
In clinical practice, most patients on anti-coagulation therapy are known to stabilize on intermediate dosages and hence, the patient populations that are known to benefit most from pre-prescription genetic testing are those at the ends of the coumarin sensitivity spectrum i.e., the hyper-and normal sensitivity groups (54%) in whom the optimum anti-coagulation may be achieved with minimal dosages or higher than average dosages, respectively. Additionally, testing will also identify the 55.6% subjects (hyper-and moderately sensitive groups) who are at an elevated risk of drug-induced adverse effects. For future research, a more systematic, comparative study with larger cohort of individuals with and without inherited thrombophilia, prospectively treated with oral anti-coagulants, is required to explore drug response with actual maintenance dose and prevalence of adverse drug reactions in the two groups. The current study provides preliminary evidence for the need of pre-prescription genotyping in patients in whom oral anti-coagulant therapy may be necessary either due to the inherited predisposition to thrombophilia or due to other thrombophilic risk factors.
 Acknowledgments Acknowledgments | |  |
We would like to thank the technical staff of the Molecular Genetics Laboratory at Center of Medical Genetics, Sir Ganga Ram Hospital for their co-operation.
 References References | |  |
| 1. | Bick RL, Kaplan H. Syndromes of thrombosis and hypercoagulability. Med Clin N Am 1998;82:409-58. 
|
| 2. | Lee AD, Stephen E, Agarwal S, Premkumar P. Venous thromboembolism in India. Eur J Vas Endovasc Surg 2009;37:482-5. 
|
| 3. | Grody WW, Griffin JH, Taylor AK, Bruce R, Korf BR, Heit JA. (ACMG Factor V Leiden Working Group). American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001;3:139-48. 
|
| 4. | College of American Pathologists′ Consensus Panel on Thrombophilia. Arch Pathol Lab Med 2001;126:1277-433. 
|
| 5. | Angeline T, Bentley HA, Hawk AB, Manners RJ, Mokashi HA, Jeyaraj N, et al. Prevalence of the Factor V G1691A and the Factor II/prothrombin G20210A gene polymorphisms among Tamilians. Exp Mol Pathol 2005;79:9-13. 
|
| 6. | Herrmann FH, Koesling M, Schrôder W, Altman R, Jiménez Bonilla R, Lopaciuk S, et al. Prevalence of Factor V Leiden Mutation in Various Populations. Genet Epidemiol 1997;14:403-11. 
|
| 7. | Middeldorp S, Meinardi JR, Koopman MM, van Pampus EC, Hamulyák K, van Der Meer J, et al. A prospective study of asymptomatic carriers of the factor V Leiden mutation to determine the incidence of venous thromboembolism. Ann Intern Med 2001;135:322-7. 
|
| 8. | Segal JB, Brotman DJ, Necochea AJ, Emadi A, Samal L, Wilson LM, et al. Predictive value of factor V leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation-A systemic review. J Am Med Assoc 2009;301:2472-85. 
|
| 9. | Taylor AK. Molecular Genetic Testing in Mainstream Medicine. Venous Thrombosis and the Factor V (Leiden) Mutation. Genetic Drift. 1997 [Internet]. Available from: http://www.mostgene.org/gd/gdvol14b.htm. [Last accessed on 2012 Mar 16]. 
|
| 10. | Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ and for the American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive Summary: Antithrombotic Therapy and Prevention of Thrombosis, 9 th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:7-47. 
|
| 11. | Pattanaik S, Dhamija P, Malhotra S, Sharma N, Pandhi P. Evaluation of cost of treatment of drug-related events in a tertiary care public sector hospital in Northern India: A prospective study. Br J Clin Pharmacol 2009;67:363-9. 
|
| 12. | Patel KJ, Kedia MS, Bajpai D, Mehta SS, Kshirsagar NA, Gogtay NJ. Evaluation of the prevalence and economic burden of adverse drug reactions presenting to the medical emergency department of a tertiary referral centre: A prospective study. BMC Clin Pharmacol 2007;7:8. 
|
| 13. | Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18820 patients. BMJ 2004;3;329:15-9. 
|
| 14. | Coumadin Tablets (Warfarin Sodium) drug label and medication guide (revised 2010). Bristol Myers Squibb Company, Princeton, New Jersey, USA. Approved by U.S.A, Food and Drug Administration. Pages 1-33. 
|
| 15. | Wadelius M, Chen LY, Eriksson N, Bumpstead S, Ghori J, Wadelius C, et al. Association of warfarin dose with genes involved in its action and metabolism. Hum Genet 2007;121:23-34. 
|
| 16. | Takahashi H, Wilkinson GR, Nutescu EA, Morita T, Ritchie MD, Scordo MG, et al. Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese, Caucasians and African-Americans. Pharmacogenet Genomics 2006;16:101-10. 
|
| 17. | Lee SC, Ng SS, Oldenburg J, Chong PY, Rost S, Guo JY, et al. Interethnic variability of warfarin maintenance requirement is explained by VKORC1 genotype in an Asian population. Clin Pharmacol Ther 2006;79:197-205. 
|
| 18. | Schalekamp T, Brassé BP, Roijers JF, Chahid Y, van Geest-Daalderop JH, de Vries-Goldschmeding H, et al. VKORC1 and CYP2C9 genotypes and acenocoumarol anticoagulation status: Interaction between both genotypes affects overanticoagulation. Clin Pharmacol Ther 2006;80:13-22. 
|
| 19. | Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 2005;352:2285-93. 
|
| 20. | Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988;16:1215. 
|
| 21. | Sproul AM, Chalmers EA. Multiplex PCR for the detection of the factor V Leiden and prothrombin 20210A mutations. In: Goulden NJ, Steward CG, Editors. Methods in Molecular Medicine: Pediatric Hematology: Methods and Protocols (Volume 91). Totowa, NJ: Humana Press; 2004. p. 79-87. 
|
| 22. | Daly AK, King BP, Leathart JB. Genotyping for cytochrome P450 polymorphisms. In: Methods in Molecular Biology. CYP450 Protocols. 2 nd ed., vol. 320. Totowa, NJ: Humana Press; 2006. p. 193207. 
|
| 23. | Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: Proposal for a new dosing regimen. Blood 2005;106:2329-33. 
|
| 24. | Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM, et al. Use of Pharmacogenetic and Clinical Factors to Predict the Therapeutic Dose of warfarin. Clin Pharmacol Ther 2008;84:326-31. 
|
| 25. | Pautas E, Moreau C, Gouin-Thibault I, Golmard JL, Mahé I, Legendre C, et al. Genetic factors (VKORC1, CYP2C9, EPHX1, and CYP4F2) are predictor variables for warfarin response in very elderly, frail inpatients. Clin Pharmacol Ther 2010;87:57-64. 
|
| 26. | Takeuchi F, McGinnis R, Bourgeois S, Barnes C, Eriksson N, Soranzo N, et al. A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet 2009;5:e1000433. 
|
| 27. | Bodin L, Verstuyft C, Tregouet DA, Robert A, Dubert L, Funck-Brentano C, et al. Cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase (VKORC1) genotypes as determinants of acenocoumarol sensitivity. Blood 2005;106:135-40. 
|
| 28. | Markatos CN, Grouzi E, Politou M, Gialeraki A, Merkouri E, Panagou I, et al. VKORC1 and CYP2C9 allelic variants influence acenocoumarol dose requirements in Greek patients. Pharmacogenomics 2008;9:1631-8. 
|
| 29. | Leung A, Huang CK, Muto R, Liu Y, Pan Q. CYP2C9 and VKORC1 genetic polymorphism analysis might be necessary in patients with Factor V Leiden and prothrombin gene G2021A mutation (s). Diagn Mol Pathol 2007;16:184-6. 
|
| 30. | Gupta PK, Ahmed RP, Bhattacharyya M, Kannan M, Biswas A, Kalra V, et al. Protein C system defects in Indian children with thrombosis. Ann Hematol 2005;84:85-8. 
|
| 31. | Günther G, Junker R, Sträter R, Schobess R, Kurnik K, Heller C, et al. Childhood Stroke Study Group. Symptomatic ischemic stroke in full term neonate: Role of acquired and genetic prothrombotic risk factors. Stroke 2000;31:2437-41. 
|
| 32. | Schobess R, Junker R, Auberger K, Münchow N, Burdach S, Nowak-Göttl U. Factor V G1691A and prothrombin G20210A in childhood spontaneous venous thrombosis-evidence of an age-dependent thrombotic onset in carriers of factor V G1691A and prothrombin G20210A mutation. Eur J Pediatr 1999;158:S105-8. 
|
| 33. | Aschka I, Aumann V, Bergmann F, Budde U, Eberl W, Eckhof-Donovan S, et al. Prevalence of factor V Leiden in children with thromboembolism. Eur J Pediatr 1996;155:1009-14. 
|
| 34. | Sharma S, Kumar SI, Poddar U, Yachha SK, Aggarwal R. Factor V Leiden and prothrombin gene G20210A mutations are uncommon in portal vein thrombosis in India. Indian J Gastroenterol 2006;25:236-9. 
|
| 35. | Garewal G, Das R, Ahluwalia J, Mittal N, Varma S. Prothrombin G20210A is not prevalent in North India. J Thromb Haemost 2003;1:2253-4. 
|
[Figure 1]
[Table 1]
| This article has been cited by | | 1 |
VKORC1 and CYP2C9 genotype distribution in Asian countries |
|
| Tejasvita Gaikwad,Kanjaksha Ghosh,Shrimati Shetty | | Thrombosis Research. 2014; | | [Pubmed] | [DOI] | | | 2 |
Possible impact of factor V Leiden genotype on warfarin induced bleeding |
|
| Gaikwad, T., Ghosh, K., Shetty, S. | | Indian Journal of Human Genetics. 2013; 19(3): 377-378 | | [Pubmed] | |
|
 |
|






