|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2013 | Volume
: 19
| Issue : 1 | Page : 9-13 |
| |
Nucleotide sequence analysis of NIPBL gene in Indian Cornelia de Lange syndrome cases
Shailesh Bajaj1, Suvidya Ranade1, Prakash Gambhir2
1 Department of Chemistry, University of Pune, Pune, Maharashtra, India
2 Birthright Genetic Clinic, Karve Road, Pune, Maharashtra, India
| Date of Web Publication | 4-Jun-2013 |
Correspondence Address:
Suvidya Ranade
Department of Chemistry, University of Pune, Pune, Maharashtra
India
 Source of Support: Department of Science and Technology, Government of India, New Delhi,, Conflict of Interest: None
PMID: 23901187 
 Abstract Abstract | | |
Background: Cornelia de Lange syndrome (CdLS) is a multisystem developmental disorder in children. The disorder is caused mainly due to mutations in Nipped-B-like protein. The molecular data for CdLS is available from developed countries, but not available in developing countries like India. In the present study, the hotspot region of NIPBL gene was screened by Polymerase Chain Reaction which includes exon 2, 22, 42, and a biggest exon 10, in six CdLS patients and ten controls.
Materials and Methods: The method adopted in present study was amplification of the target exon by using polymerase chain reaction, qualitative confirmation of amplicons by Agarose Gel Electrophoresis and use of amplicons for Conformation Sensitive Gel Electrophoresis to find heteroduplex formation followed by sequencing.
Results: We report two polymorphisms in the studied region of gene NIPBL. The polymorphisms are in the region of intron 1 and in exon 10. The polymorphism C/A is present in intron 1 region and polymorphism T/G in exon 10.
Conclusion: The intronic region polymorphism may have a role in intron splicing whereas the polymorphism in exon 10 results in amino acid change (Val to Gly). These polymorphisms are disease associated as these are found in CdLS patients only and not in controls.
Keywords: Cornelia de Lange syndrome, Indian Cornelia de Lange syndrome, NIPBL, polymorphisms
How to cite this article:
Bajaj S, Ranade S, Gambhir P. Nucleotide sequence analysis of NIPBL gene in Indian Cornelia de Lange syndrome cases. Indian J Hum Genet 2013;19:9-13 |
How to cite this URL:
Bajaj S, Ranade S, Gambhir P. Nucleotide sequence analysis of NIPBL gene in Indian Cornelia de Lange syndrome cases. Indian J Hum Genet [serial online] 2013 [cited 2016 May 24];19:9-13. Available from: http://www.ijhg.com/text.asp?2013/19/1/9/112876 |
 Introduction Introduction | |  |
Cornelia de Lange syndrome (CdLS) is a multisystem developmental disorder, which was recognized for the 1 st time in 1916 and 1933 by Brachman and de Lange respectively. The CdLS is characterized by facial dysmorphia, upper extremity malformation, hirsutism, cardiac defects, and GI abnormalities. [1] The typical features of CdLS include thin eyebrows which frequently meet at midline, long eye lashes, upturned nose and thin downturned lips, excessive body hair, small head, partial joining of 2 nd , and 3 rd toes, gastroesophageal reflux, seizures heart defects, cleft palate, bowel abnormalities and developmental delay. [1] Recently, a new finding in CdLS, bilateral split fit is reported from Turkey. [2] There are reported cases of CdLS from USA, France, Brazil, Italy, Canada, and India. The overall frequency is 1 in 10,000 live births. [3] The genes responsible for CdLS (NIPBL; Nipped-B-Like and SMC1A; Structural Maintainance of Chromosome) have been identified recently in 2004. [3],[4]
The gene NIPBL has 47 exons (46 coding) and it spans around 190 kb. The gene is located on chromosome 5p13.1. Coding sequence commences in exon 2 continues either to exon 47 generating a long isoform 2804 amino acid or to expanded variant of exon 46 generating slightly shorter isoform. NIPBL is strongly expressed in fetal and adult kidney, fetal liver, adult placenta, heart, skeletal muscle, and thymus, but weakly or almost undetectably expressed in fetal and adult brain and lung and in adult liver, colon, small intestine, and leukocytes. [4] Cohesin forms a large ring like structure composed of SMC1, SMC3, RAD21, and SA (Stromalin) proteins which are required for sister chromatid cohesion. [5] Mutation in SMC3 or SMC1A gene affects binding of Cohesin ring to DNA. [6] NIPBL helps in loading and unloading of Cohesin ring on chromatin. NIPBL and Cohesin co localize and bind preferentially to active transcriptional units. Establishment of cohesion during S phase depends on acetylation of SMC3's nucleotide-binding domain by the Eco1 acetyl transferase. SMC3 acetylation contributes to the maintenance of sister chromatid cohesion. [7] A dual role for Nipped-B in sister chromatid cohesion and developmental regulation was confirmed by Rollins et al. [8] The majority of the mutations causing CdLS are protein truncating leading to haploinsufficiency of the protein product. Tonkin et al. analyzed de novo balanced translocations associated with CdLS. [9] Total 56 mutations have been identified which include, 21 frame shift, 12 missense, 10 nonsense, and 8 splice site mutations. [9] Some de novo mutations are also reported. To date mutations in this gene have been identified in over 45-50% of individuals with CdLS. [10] No mutation has been identified in codons 4-6, 8, 11-14, 16, 19, 23-25, 30-34, 36, 37, 41, and 47. Several exons have been found to have multiple mutations including exons 2, 3, 7, 9, 10, 17, 22, 28, 29, 40, 42, and 45. [11] There has been preponderance of mutations identified in exon 10 however, this is 1625 bp exon, which is 8 times bigger than the average size of the exon. Mutations (e.g., missense, splice site, frame shift and complex) are spread through out the gene, although there appears to be some clustering of mutations in exon 10. [11] Tonkin et al. studied CdLS cases without translocations and identified nine plausible point mutations, at least five of which arose by de novo. [9] Yan et al. identified 13 different NIPBL mutations, including 11 novel mutations. [12] Recently, it has been reported that mutations in NIPBL or Cohesion results in transcriptional dysregulation. [13] The mutations causing CdLS have been identified in different parts of the world. There is no data available for the mutations in NIPBL gene neither from Indian patients nor from the Maharashtra region. With the objective to identify the mutations in NIPBL gene in the Indian patients with CdLS the present work is undertaken. This will generate molecular data in these patients and the diagnosis of disease at molecular level would be established as routine method.
 Materials and Methods Materials and Methods | |  |
Materials
Patients of clinically, diagnosed CdLS along with their family and control were studied. Patients consent form and family information form was filled for each patient. Six families along with ten controls were studied. Five milliliter blood of control, patient and parents were collected.
Chemicals and equipments
DNA isolation and polymerase chain reaction (PCR) purification kits (QIAGEN), PCR chemicals, hot start Taq DNA polymerase (Bangalore genei), Thermal cycler (Eppendorf).
Methods
Preliminary study
The details of the family history was taken for the case studied as follows: Name, age, address, community, pedigree, and family history of CdLS, consanguinity, and clinical phenotype. DNA of the patient along with the parents and control was isolated from 5 ml of peripheral blood using a kit from QIAGEN.
Amplification of NIPBL using specific primers
The exon 10, which is the biggest exon and hotspot region of the NIPBL gene, was amplified using 6 different primers. Along with these exons 2, 22, and 42 were also studied. Cycling parameters were as follows: 36 cycles of: 94°C - 30 s; 55-62°C - 45 s, 72°C - 30 s; 72°C for 5 min final extension. The primer sequences were obtained from the study carried out by Gillis et al. 2004. [11] [Table 1] shows primer sequences along with product size. New primer set for intron 1 has been developed by author for this study.
The reaction was constituted with 75 ng genomic DNA, 1U Taq Polymerase, primers forward and reverse 20 pmol/μl, Buffer 1X, dNTPs 75 μ mol each, 1.5 mM MgCl 2 and sterile water for dilution.
Analysis of specific product using conformation sensitive gel electrophoresis
The PCR Products for the above reaction was run on CSGE to check altered mobility due to heteroduplex formation. [14]
DNA sequencing
Those PCR products, which showed multiple bands on CSGE were purified using PCR purification kit and sequenced using ABI 3730 DNA analyzer. The sequencing of the amplified product was carried out at National Centre for Cell Science, Pune University and Geneombio Laboratory privately. Sequencing was carried out in forward and reverse direction for at least three times. The base change and position was identified from sequenced data using bioinformatics tools.
 Results Results | |  |
The patients studied were phenotypically shown the following features: Mental retardation, short stature, hypertrichosis, presence of microcephaly, synophrys, low antiriot hairline, narrow forehead, hoarse voice, marked on back, restricted movements of elbows, bilateral simian crease, short little finger with clinodactyly, hypoplastic nipples, shield chest, shawl scrotum, micropenis, and small testes. Family history for disease was nil. The families of these CdLS patients were studied for mutational analysis using PCR. The patients DNA isolated from leukocytes were subjected to amplification using primers for exons 2, 10, 22, and 42. [Figure 1] shows the separation of amplicons on Agarose Gel Electrophoresis.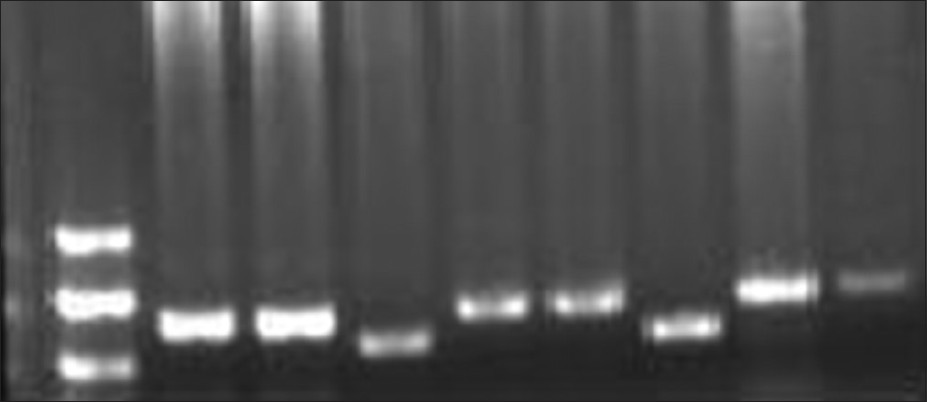 | Figure 1: Well No 1-100 bp ladder, 2-7 amplified product exon 10 (A-F primers) well 8 and 9 - exon 2 and 42
Click here to view |
The amplified products of exon 2, 22, 42, and 10 were run on CSGE to identify the presence of heteroduplexes due to DNA base changes. Amplified product of exon 2 and 10B showed presence of heteroduplex on CSGE. It showed presence of multiple bands on CSGE. Purified PCR products for exon 2 and 10B were given for sequencing. After sequencing of the amplicons for exon 2 and 10B, the sequences were used and nucleotide BLAST was carried out to find a mismatched nucleotide. In case of amplified product of exon 2, the base change was found in the upstream region of exon 2. The BLAST results showed presence of polymorphism C/A in intron 1 at 36943536 in family number three and in three other patients [Figure 2]. The primer pair for exon 2 partially covers region of intron 1. This change was present in the primer binding region of forward primer and was difficult for sequencing by using the primer sequence of Gillis et al. Thus, new primers were designed by authors. These primers kept this polymorphic position in center of amplicon product so that one could easily sequence it. These primers were annealing in intron 1 region. The size of the new amplicon was 220 bp. This polymorphic site containing region is important to splice out the intron 1 thus, the polymorphism may have some role in splicing. The polymorphism at same site was found in patient's brother also. The heterozygous condition for the trait is found in family. Control did not show any polymorphism at same position. These results were confirmed by sequencing of amplified PCR product three times in both directions.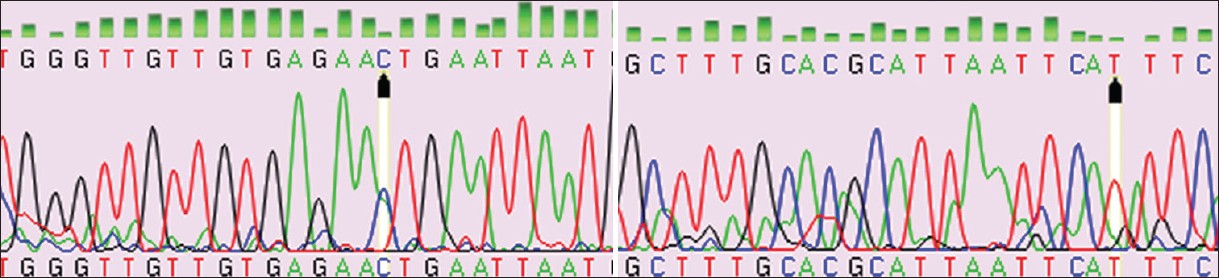 | Figure 2: Exon 2 fwd chromatogram (patient 3rd) exon 2 rev chromatogram (patient 3rd)
Click here to view |
The second polymorphism T/G is found in exon 10 at 36975174, mRNA position 2391 [Figure 3]. This polymorphism was also in heterozygous condition in family three. The polymorphism was in the coding region of gene NIPBL hence affects the codon, which ultimately changes the amino acid in polypeptide. This polymorphism was seen in patient, parents and her brother in family number three.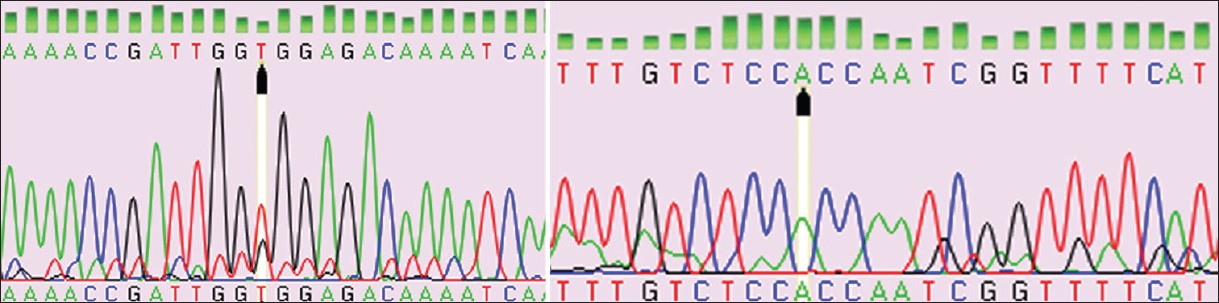 | Figure 3: Exon 10B fwd chromatogram (patient 3rd) exon 10B rev chromatogram (patient 3rd)
Click here to view |
The amino acid changed due to this polymorphism is Valine to Glycine at position 631 of nipped B. The control DNA was also amplified and studied in a similar way. There has been no report of polymorphism in the studied region and protein position 631 in literature. However, the recent report mentions the mutation due to deletion resulting in Gly-Val substitution at protein positions 2268 and 1589 with unknown phenotype. The physicochemical property of Gly and Val are different and it might affect protein function and binding up to certain extent.
 Discussion Discussion | |  |
We have studied the NIPBL gene at molecular level for the 1 st time to identify the nucleotide sequence changes in Indain families. The gene NIPBL is composed of 47 exons, of which 46 are coding. The polymorphism C/A which is at position 36943536 is in the intron 1 region. The polymorphism in this region may affect splicing of the intron 1. The usual base present at position 36943536 on the forward and reverse strand is C and G respectively. The polymorphism C/A is present on forward strand where as the bases on reverse strand were found to be G to T. T is complementary to A, so on some copies the base pairing is correct but some alleles carry C on forward and T on reverse strand, which is incorrect matching of base. Borck et al. has also reported similar result in upstream region of NIPBL gene. [15]
Another polymorphism was found in exon 10 is in the coding region and changes amino acid Valine to Glycine. The codon changes due to polymorphism T/G is GTG to GGG. The proteins formed in patient and family members may contain either Valine or Glycine in protein. If we consider the effect of amino acid change on the protein functioning, it may not be much severe as amino acids have different physicochemical properties.
This is the first report of molecular studies on Indian patients and these are the polymorphisms, the family members and patient have one normal allele along with polymorphic allele. Functioning of one normal allele is also sufficient for normal gene expression thus it may not be directly responsible for CdLS and gene expression. The unaffected parents giving birth to children with CdLS could be the result of germ line mosaicism. Similar explanation can be given for children having NIPBL mutations born to mutation negative parents. The present study aims at screening the entire NIPBL coding region for mutation identification. At present, we have screened hotspot region and found two different base changes, but these were found to be polymorphisms and not actual mutations. Heterozygous parents having homozygous patients may be the disease causing factor up to certain extent. Further study will be undertaken to screen entire gene by using a robust gene screening technique like Multiplex Ligation-dependent Probe Amplification.
Since, we have found polymorphism in Indian patient for the 1 st time the sequence will be deposited to Single Nucleotide Polymorphism database.
 Acknowledgments Acknowledgments | |  |
We are very thankful to the patients and families, for generously donating samples and clinical information. We are also thankful to Department of Science and Technology for providing funding, Dr. A. A. Kumbhar for providing gel documentation facility, National Centre for Cell Science, University of Pune, Dr. Shouche's group for providing sequencing facility, and Department of Chemistry for providing infrastructure and other facilities.
 References References | |  |
| 1. | Jackson L, Kline AD, Barr MA, Koch S. de Lange syndrome: A clinical review of 310 individuals. Am J Med Genet 1993;47:940-6. 
|
| 2. | Dogan DG, Dogan M, Aslan M, Karabiber H. Bilateral split feet: A new finding in Cornelia de Lange syndrome. Genet Couns 2010;21:221-4. 
|
| 3. | Krantz ID, McCallum J, DeScipio C, Kaur M, Gillis LA, Yaeger D, et al. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat Genet 2004;36:631-5. 
|
| 4. | Tonkin ET, Wang TJ, Lisgo S, Bamshad MJ, Strachan T. NIPBL, encoding a homolog of fungal Scc2-type sister chromatid cohesion proteins and fly Nipped-B, is mutated in Cornelia de Lange syndrome. Nat Genet 2004;36:636-41. 
|
| 5. | Gruber S, Haering CH, Nasmyth K. Chromosomal cohesin forms a ring. Cell 2003;112:765-77. 
|
| 6. | Revenkova E, Focarelli ML, Susani L, Paulis M, Bassi MT, Mannini L, et al. Cornelia de Lange syndrome mutations in SMC1A or SMC3 affect binding to DNA. Hum Mol Genet 2009;18:418-27. 
|
| 7. | Beckouët F, Hu B, Roig MB, Sutani T, Komata M, Uluocak P, et al. An Smc3 acetylation cycle is essential for establishment of sister chromatid cohesion. Mol Cell 2010;39:689-99. 
|
| 8. | Rollins RA, Korom M, Aulner N, Martens A, Dorsett D. Drosophila nipped-B protein supports sister chromatid cohesion and opposes the stromalin/Scc3 cohesion factor to facilitate long-range activation of the cut gene. Mol Cell Biol 2004;24:3100-11. 
|
| 9. | Tonkin ET, Smith M, Eichhorn P, Jones S, Imamwerdi B, Lindsay S, et al. A giant novel gene undergoing extensive alternative splicing is severed by a Cornelia de Lange-associated translocation breakpoint at 3q26.3. Hum Genet 2004;115:139-48. 
|
| 10. | Barisic I, Tokic V, Loane M, Bianchi F, Calzolari E, Garne E, et al. Descriptive epidemiology of Cornelia de Lange syndrome in Europe. Am J Med Genet A 2008;146A: 51-9. 
|
| 11. | Gillis LA, McCallum J, Kaur M, DeScipio C, Yaeger D, Mariani A, et al. NIPBL mutational analysis in 120 individuals with Cornelia de Lange syndrome and evaluation of genotype-phenotype correlations. Am J Hum Genet 2004;75:610-23. 
|
| 12. | Yan J, Saifi GM, Wierzba TH, Withers M, Bien-Willner GA, Limon J, et al. Mutational and genotype-phenotype correlation analyses in 28 Polish patients with Cornelia de Lange syndrome. Am J Med Genet A 2006;140:1531-41. 
|
| 13. | Liu J, Zhang Z, Bando M, Itoh T, Deardorff MA, Clark D, et al. Transcriptional dysregulation in NIPBL and cohesin mutant human cells. PLoS Biol 2009;7:e1000119. 
|
| 14. | Ganguly A, Rock MJ, Prockop DJ. Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double-stranded PCR products and DNA fragments: Evidence for solvent-induced bends in DNA heteroduplexes. Proc Natl Acad Sci U S A 1993;90: 10325-9. 
|
| 15. | Borck G, Zarhrate M, Cluzeau C, Bal E, Bonnefont JP, Munnich A, et al. Father-to-daughter transmission of Cornelia de Lange syndrome caused by a mutation in the 5' untranslated region of the NIPBL Gene. Hum Mutat 2006;27:731-5. 
|
[Figure 1], [Figure 2], [Figure 3]
[Table 1]
|






