|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2013 | Volume
: 19
| Issue : 2 | Page : 154-158 |
| |
Haptoglobin2-2 phenotype is an additional risk factor of retinopathy in type 2 diabetes mellitus
Mukund R Mogarekar, Mahesh H Hampe
Department of Biochemistry, S. R. T. R. Medical College, Ambajogai, Maharashtra, India
| Date of Web Publication | 5-Aug-2013 |
Correspondence Address:
Mukund R Mogarekar
Department of Biochemistry, S. R. T. R. Medical College, Ambajogai, District Beed, Maharashtra
India
 Source of Support: None, Conflict of Interest: None
DOI: 10.4103/0971-6866.116111

 Abstract Abstract | | |
Aims: The aim of this study was to investigate the association between haptoglobin (Hp) phenotypes and risk of the development of diabetic retinopathy (DR) in patients of type 2 diabetes mellitus.
Materials and Methods: This cross-sectional study included 45 normotensive type 2 diabetic patients (duration more than 5 years) admitted in the hospital divided into two groups (with and without DR) on the basis of fundus examination by direct ophthalmoscopy. Serum samples of all patients were subjected for Hp phenotyping by polyacrylamide gel electrophoresis.
Results: DR was associated significantly in diabetic patients with Hp2-2 phenotype (79.31%) than diabetic patients with Hp2-1 phenotype (43.75%) and Hp2-2 had higher odds ratio (OR) for DR in univariate analysis (OR 4.929, [95% confidence interval [CI] (1.297-18.733)], P = 0.016) and multivariate analysis (OR 7.704 [95% CI (0.887-66.945)], P = 0.064). Furthermore, Hp2-2 was associated significantly with severe forms of DR.
Conclusion: Hp2-2 phenotype is associated with susceptibility to DR showing a graded risk relationship to the number of Hp2 alleles. Determination of Hp phenotype may be useful in the risk assessment and management of DR.
Keywords: Diabetes mellitus, diabetic retinopathy, haptoglobin
How to cite this article:
Mogarekar MR, Hampe MH. Haptoglobin2-2 phenotype is an additional risk factor of retinopathy in type 2 diabetes mellitus. Indian J Hum Genet 2013;19:154-8 |
How to cite this URL:
Mogarekar MR, Hampe MH. Haptoglobin2-2 phenotype is an additional risk factor of retinopathy in type 2 diabetes mellitus. Indian J Hum Genet [serial online] 2013 [cited 2016 May 24];19:154-8. Available from: http://www.ijhg.com/text.asp?2013/19/2/154/116111 |
 Introduction Introduction | |  |
Diabetes mellitus (DM) is a major health problem world-wide and diabetic retinopathy (DR) is the most common and severe chronic ocular microvascular complication eventually leading to blindness. [1],[2],[3] The prevalence of DR in diabetic individuals is around 17% in India amongst which around 8% ends in vision threatening blindness. [4],[5] The risk of retinopathy is directly related to the age at onset of hyperglycemic state, degree of glycemic control and duration of diabetes. [6],[7],[8] Several hypotheses have been proposed to explain the mechanism for the development of retinopathy in DM such as polyol accumulation, formation of advanced glycation end products, oxidative stress and activation of protein kinase C modulating the disease process through various cellular metabolic signaling. [9],[10],[11],[12],[13],[14]
Haptoglobin (Hp) is a positive acute phase reactant with hemoglobin (Hb) binding capacity. [15] Free Hb is considered to be a potent pro-oxidant which mediate several oxidative pathways like Fenton reaction resulting in the formation of hydroxyl radicals. [16] Hp binds with Hb and removes it from the circulation and thus prevents iron catalyzed formation of oxygen radicals and thus playing an important role as an antioxidant. [17] Hp-Hb binding also inhibit prostaglandin synthesis by removing heme compounds which catalyze oxidation of arachidonic acid by prostaglandin synthase, thus exerting anti-inflammatory effect. [18] Hp binding depends not only on serum Hp concentration but also on Hp phenotype. [15] In humans, the Hp gene is located on the long arm of chromosome 16. [19] It consists of two alleles, Hp1 and Hp2 which manifest as three phenotypes, Hp1-1, Hp2-1 and Hp2-2. The increased antioxidant function of Hp1-1 is thought to offer better protection from the vascular damage while Hp2-2 is believed to be a major risk factor in several oxidative stress related disease states. [19],[20]
Thus Hp polymorphism offers differential protection towards oxidative stress induced microvascular damage in genetically susceptible individuals. This gave us the insight to investigate diabetic patients to determine the association of DR with Hp phenotypes. The aim of the present study was to investigate the association between Hp phenotypes and occurrence of retinopathy in Type 2 diabetic patients.
 Materials and Methods Materials and Methods | |  |
Study was conducted on 45 normotensive Type 2 DM patients diagnosed as per the diagnostic criteria of American Diabetes Association, 2007 selected randomly from the patients admitted in hospital wards. Diabetic patients with retinopathy formed the study group while diabetic patients without retinopathy served as the control group. [21] Study protocol was approved by the institutional ethics committee. Written informed consent was obtained from all patients. Patients with associated ischemic heart disease, rheumatoid arthritis, concomitant liver or kidney disease, history of exogenous hormone administration and DM of less than 5 years duration were excluded from the study population.
Fundus examination
Fundus examination of both eyes was carried out by direct ophthalmoscopy 30 min after complete mydriasis with 1% tropicamide eye drops. Indirect ophthalmoscopy with +20 D lens and examination of macula with +90 D Volk's lens was carried out whenever indicated. Grading of DR was carried out according to Early Treatment Diabetic Retinopathy Study criteria. [7]
All cases included in this study were subjected to detailed history taking, a complete clinical examination and abdominal ultrasonography. Height and weight were measured in barefoot patients wearing only light clothes. Body mass index was calculated by the Quetelet's index, Weight (kg)/height 2 (m).
Analysis of serum and plasma samples
All fine chemicals of analytical grade were obtained from Sigma or Merck, India. Venous blood sample was collected aseptically from all patients in the morning after an overnight fast. Fasting blood sample collected in fluoride bulb was analyzed within a few hours for estimation of plasma glucose by glucose oxidase-peroxidase method. [22] Serum was collected from remaining blood sample by low speed centrifugation and was subjected to various biochemical analyses. Levels of total cholesterol (TC), High density lipoprotein (HDL) cholesterol and triglycerides were measured using enzymatic techniques (Erba diagnostic kit). Low density lipoprotein (LDL) cholesterol was calculated by the Friedewald formula. [23] Total oxidant status (TOS) was estimated by the method as described by Ozcan Erel and expressed as μmol H 2 O 2 equivalents/L. [24]
Haptoglobin phenotyping
Hp phenotype distribution was determined by using 7% polyacrylamide gel electrophoresis (PAGE) as previously described by Hochberg et al. [25]
Statistical analysis
Hardy-Weinberg equilibrium was evaluated by means of the χ2 test. The continuous variables were tested for normality with Shapiro-Wilk test. Results were expressed as mean ± SD. Comparisons between groups were made using Student's t-test for continuous variables. Pearson χ2 and Fisher's exact test were performed to compare the frequencies of Hp phenotypes between subjects with and without retinopathy. Initially variables including known risk factors for factors for diabetes were assessed in univariate analysis and then they are modeled using stepwise multivariate logistic regression analysis to determine the additional risk factors of DR. Hp polymorphism was then added to subsequent model that controlled for the major risk factors for DR. Naglekerke R 2 was compared for each of the logistic regression models. Statistical significance was considered at the level of P < 0.05. All tests were performed with the statistical software Mystat 12 for Windows.
 Results Results | |  |
The main clinical and biochemical data of diabetics is shown [Table 1]. Fundus examination revealed that 15 diabetic patients had no signs of DR, 24 diabetics had mild to moderate DR while 6 diabetics had severe non-proliferative DR. Patients with DR were found to have longer duration of disease and statistically significant difference in fasting blood glucose levels (P = 0.007). Age, sex, triglyceride, TC, HDL cholesterol, very low density lipoprotein cholesterol, LDL cholesterol and TOS were statistically insignificant in the two groups. The three phenotypes (Hp1-1, Hp2-1 and Hp2-2) in diabetic patients were easily distinguished by a characteristic pattern of bands representing the Hp-Hb complex [Figure 1]. The distribution of allele frequencies for Hp phenotypes was not in Hardy-Weinberg equilibrium. This could be because of selection bias which included only diabetic cases and small sample size (n = 45). We found no significant difference between the Hp phenotypes for any of the diabetes risk factors as determined by both univariate analysis or by multivariate logistic regression analysis (data not shown).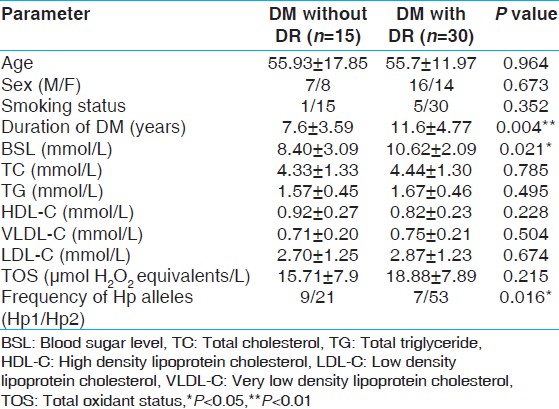 | Table 1: Clinical and biochemical data of type 2 diabetic patients with and without retinopathy
Click here to view |
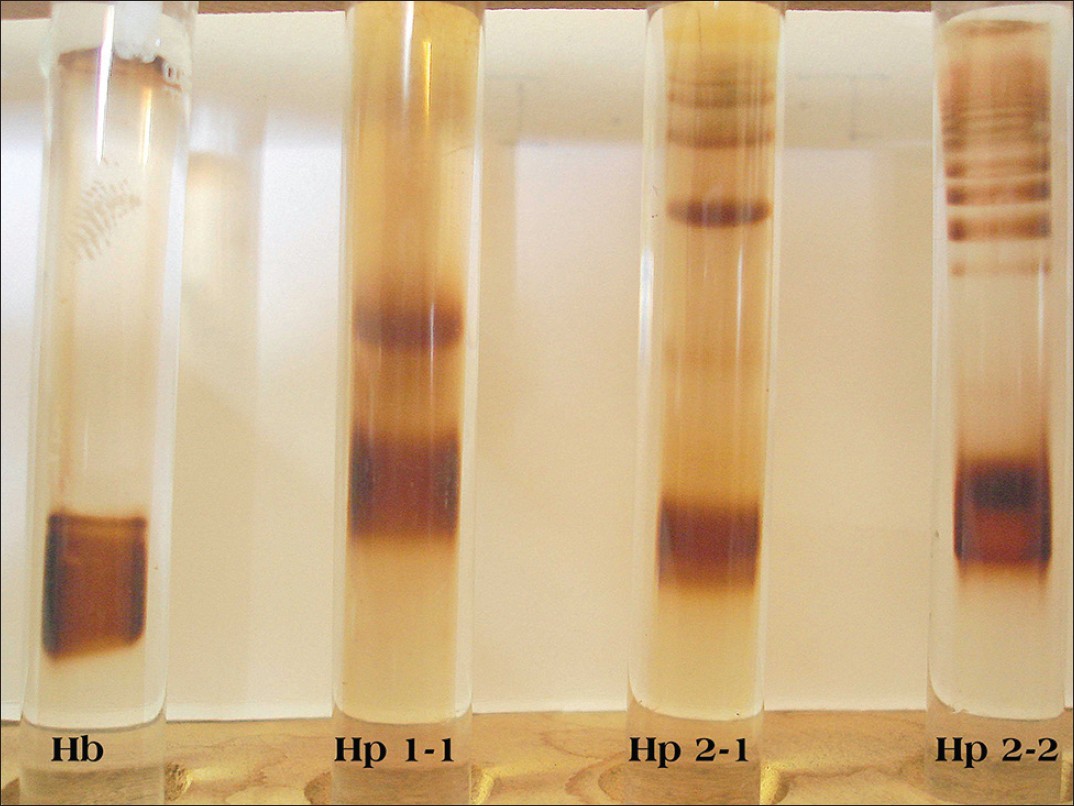 | Figure 1: Characteristic pattern of bands of three haptoglobin phenotypes after polyacrylamide gel electrophoresis. Bands correspond to Hp-hemoglobin complexes. The most distal band in each tube represents unbound Hb. Hp phenotypes: First tube Hb band, second tube Hp1-1, third tube Hp2-1 and fourth tube Hp2-2
Click here to view |
[Table 2] summarizes the distribution of the Hp allele frequencies in the patients studied according to DR status. Among the 45 diabetics included in the study, none of the patients had Hp1-1 phenotype. 7 of 16 diabetic patients (43.75%) with Hp2-1 and 23 out of 29 diabetic patients (79.31%) with Hp2-2 had evidence of DR. The difference in the occurrence of DR between patients with Hp2-1 and Hp2-2 phenotype was statistically significant. Moreover, the frequency of patients with Hp2-2 phenotype was greater in diabetics with severe forms of DR. As determined by univariate logistic regression, patients with DM and Hp2-2 were more likely to have had DR than those with DM and Hp2-1 phenotype. (Odds ratio (OR) 4.293, (95% confidence interval (CI) (1.297-18.733)), P = 0.016). This data suggests greater risk associated with Hp2 allele in the development of DR in patients of DM. Out of the established biochemical and clinical characteristics, duration of disease and advanced age were the only independent predictors of DR in our study group [Table 3]. In the logistic regression models prepared for Hp2-2 phenotype as a predictor of increased risk of DR, the significant association between Hp2-2 and DR identified in univariate testing remained borderline significant in multivariate testing (model 2). The increased risk of DR predicted by the Hp2-2 phenotype is estimated after adjustment of major risk factors of DR. (OR 7.704 (95% CI (0.887-66.945)), P = 0.064). Moreover, Naglekerke R 2 value increased after addition of Hp phenotyping in the model prepared by the conventional risk factors of DM, indicating independent association of Hp polymorphism with occurrence of DR.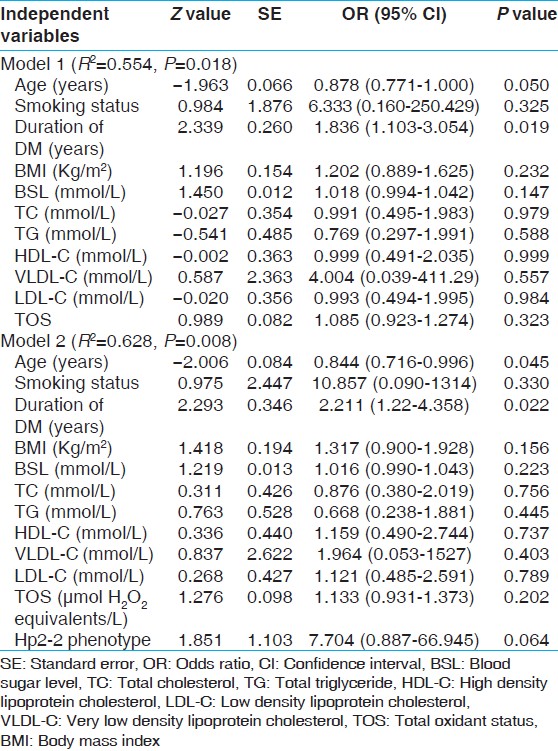 | Table 3: Multivariate models of predictors of retinopathy in diabetic patients
Click here to view |
 Discussion Discussion | |  |
DR is the most common and serious microvascular complication in both type 1 and type 2 DM. [26] Duration of diabetes and degree of glycemic control are the two major determinants in the development of retinopathy. [3],[27] However these are not the only sufficient conditions which explain the development of microvascular complications. The occurrence of retinopathy even in strict glycemic control and short duration of diabetes evokes possibility of non-modifiable genetic factors which contribute to the development of DR. Genetically determined differences in antioxidant protection could contribute to differential susceptibility of diabetic patients to microvascular complications. One such hepatically synthesized protein is Hp which shows genetic variability determined by two alleles, Hp1 and Hp2, which manifests as three phenotypes. [28] We performed Hp phenotyping by PAGE. In our study, Hp2-2 phenotype was more associated with development and progression of retinopathy in diabetic patients. Moreover, Hp2-2 phenotype had more severe forms of DR. Hp2-2 phenotype had higher OR for the development of DR than the diabetics with Hp2-1 phenotype. This relationship persisted even after adjustment of conventional DM characteristics. Thus Hp2 allele has been found to be an additional risk predictor for the development of retinopathy in diabetic individuals. Our findings are consistent with the study conducted by Nakhoul et al. (2000) which demonstrated a graded risk relation between presence, as well as severity, of DR and Hp phenotype. [29] Nakhoul et al.(2001) and Bessa et al .(2007) studied Hp polymorphic distribution in type 2 diabetic patients and demonstrated that Hp2-2 is a major susceptibility gene for development of DR. [30],[31] In the majority of studies, persons with Hp2-2 were found to be at increased risk of microvascular complications. [20],[28] However two other studies by Ratzman et al.(1984) and Koda et al. (2002), which compared diabetic patients with and without DR and found no difference in Hp phenotype distribution in two groups. [32],[33] The reasons for these discrepancies may be due to differences in type of diabetes, ethnic groups and other genetic contributors and also the collected data is relatively small.
Hp exerts its protective effect by working as Hb scavenger and an antioxidant. It binds with Hb with high affinity and removes it from the circulation and thus prevents iron mediated formation of free radicals. [34],[35] Furthermore Hp levels are remarkably correlated with phenotypes. Individuals with Hp2-2 had the lowest levels of haptoglobin, those with Hp2-1 had intermediate levels and those with Hp1-1 had the highest levels. [3] Hp-Hb binding is also phenotype-dependent. [17] Because of poor sieving of highly polymeric Hp 2-2 protein due to its large molecular mass, it exerts less efficient antioxidative capacity. [36] These unfavorable characteristics of Hp2-2 phenotype may explain the significant association of retinopathy in diabetic patients with Hp2-2 phenotype. However, the limitation to our study is the small sample size, which is not enough to establish the predictive value of Hp polymorphism in DR and larger studies are required to serve this purpose. Furthermore, residual confounding by the variables cannot be accounted.
This study, therefore gives us an alarm for more energetic treatment for retinopathy in diabetic patients with Hp2-2 phenotype. Furthermore, Hp phenotype can be considered as a possible candidate that can be used in the algorithm followed for the management of cases of DR.
 References References | |  |
| 1. | Lamoureux EL, Wong TY. Diabetic retinopathy in 2011: Further insights from new epidemiological studies and clinical trials. Diabetes Care 2011;34:1066-7. 
[PUBMED] |
| 2. | Ferris FL 3 rd , Davis MD, Aiello LM. Treatment of diabetic retinopathy. N Engl J Med 1999;341:667-78. 
|
| 3. | Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Diabetic retinopathy. Diabetes Care 2003;26:S99-S102. 
[PUBMED] |
| 4. | Rema M, Pradeepa R. Diabetic retinopathy: An Indian perspective. Indian J Med Res 2007;125:297-310. 
[PUBMED]  |
| 5. | Agrawal RP, Ranka M, Beniwal R. Gothwal SR, Jain GC, Kochar DK et al. Prevalence of diabetic retinopathy in type 2 diabetes in relation to risk factors: Hospital based study. Int J Diabetes Dev Ctries 2003;23:16-9. 
|
| 6. | Balsubramanyam M, Rema M, Premanand C. Biochemical and molecular mechanisms of diabetic retinopathy. Curr Sci 2002;83:1506-14. 
|
| 7. | Dharmalingam M. Diabetic retinopathy-Risk factors and strategies in prevention. Int J Diabetes Dev Ctries 2003;11:10-3. 
|
| 8. | Domínguez C, Ruiz E, Gussinye M, Carrascosa A. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care 1998;21:1736-42. 
|
| 9. | Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care 1996;19:257-67. 
[PUBMED] |
| 10. | Fong DS, Aiello LP, Ferris FL 3 rd , Klein R. Diabetic retinopathy. Diabetes Care 2004;27:2540-53. 
|
| 11. | Frank RN. Diabetic retinopathy. N Engl J Med 2004;350:48-58. 
[PUBMED] |
| 12. | Ramakrishna V, Jailkhani R. Evaluation of oxidative stress in Insulin Dependent Diabetes Mellitus (IDDM) patients. Diagn Pathol 2007;2:22. 
[PUBMED] |
| 13. | Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 2004;18:1450-2. 
[PUBMED] |
| 14. | Cohen O, Norymberg K, Neumann E, Dekel H. Complication-free duration and the risk of development of retinopathy in elderly diabetic patients. Arch Intern Med 1998;158:641-4. 
[PUBMED] |
| 15. | Nielsen MJ, Moestrup SK. Receptor targeting of hemoglobin mediated by the haptoglobins: Roles beyond heme scavenging. Blood 2009;114:764-71. 
[PUBMED] |
| 16. | Everse J, Hsia N. The toxicities of native and modified hemoglobins. Free Radic Biol Med 1997;22:1075-99. 
[PUBMED] |
| 17. | Szafranek T, Marsh S, Levy AP. Haptoglobin: A major susceptibility gene for diabetic vascular complications. Exp Clin Cardiol 2002;7:113-9. 
|
| 18. | Jue DM, Shim BS, Kang YS. Inhibition of prostaglandin synthase activity of sheep seminal vesicular gland by human serum haptoglobin. Mol Cell Biochem 1983;51:141-7. 
[PUBMED] |
| 19. | Wobeto VP, Zaccariotto TR, Sonati MF. Polymorphism of human haptoglobin and its clinical importance. Genet Mol Biol 2008;31:602-20. 
|
| 20. | Dobryszycka W. Haptoglobin in the new millennium. Adv Clin Exp Med 2004;13:3-18. 
|
| 21. | American Diabetes Association. Diagnosis and classification of diabetic retinopathy. Diabetes care 2007;30:S42-47. 
[PUBMED] |
| 22. | Cramp DG. New automated method for measuring glucose by glucose oxidase. J Clin Pathol 1967;20:910-2. 
[PUBMED] |
| 23. | Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502. 
[PUBMED] |
| 24. | Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem 2005;38:1103-11. 
[PUBMED] |
| 25. | Hochberg I, Roguin A, Nikolsky E, Chanderashekhar PV, Cohen S, Levy AP. Haptoglobin phenotype and coronary artery collaterals in diabetic patients. Atherosclerosis 2002;161:441-6. 
[PUBMED] |
| 26. | Sihota R, Tandon R. Diseases of the retina. In: Sihota R, editor. Parsons' Diseases of the Eye. 21 st ed. New Delhi: Elsevier Health Sciences; 2011. p. 305-10. 
|
| 27. | Sun JK, Keenan HA, Cavallerano JD, Asztalos BF, Schaefer EJ, Sell DR, et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: The joslin 50-year medalist study. Diabetes Care 2011;34:968-74. 
|
| 28. | Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem 1996;42:1589-600. 
|
| 29. | Nakhoul FM, Marsh S, Hochberg I, Leibu R, Miller BP, Levy AP. Haptoglobin genotype as a risk factor for diabetic retinopathy. JAMA 2000;284:1244-5. 
|
| 30. | Nakhoul FM, Zoabi R, Kanter Y, Zoabi M, Skorecki K, Hochberg I, et al. Haptoglobin phenotype and diabetic nephropathy. Diabetologia 2001;44:602-4. 
|
| 31. | Bessa SS, Hamdy SM, Ali EM. Haptoglobin gene polymorphism in type 2 diabetic patients with and without nephropathy: An Egyptian study. Eur J Intern Med 2007;18:489-95. 
|
| 32. | Ratzmann KP, Strese J, Keilacker H, Giebelmann R, Scheibe F, Witt S. Is there a relationship between genetically determined haptoglobin phenotype and insulin-dependent diabetes mellitus (IDDM). Exp Clin Endocrinol 1984;83:207-15. 
|
| 33. | Koda Y, Soejima M, Yamagishi S, Amano S, Okamoto T, Inagaki Y, et al. Haptoglobin genotype and diabetic microangiopathies in Japanese diabetic patients. Diabetologia 2002;45:1039-40. 
|
| 34. | Theilgaard-Mönch K, Jacobsen LC, Nielsen MJ, Rasmussen T, Udby L, Gharib M, et al. Haptoglobin is synthesized during granulocyte differentiation, stored in specific granules, and released by neutrophils in response to activation. Blood 2006;108:353-61. 
|
| 35. | Levy AP, Hochberg I, Jablonski K, Resnick HE, Lee ET, Best L, et al. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The strong heart study. J Am Coll Cardiol 2002;40:1984-90. 
|
| 36. | Sadrzadeh SM, Bozorgmehr J. Haptoglobin phenotypes in health and disorders. Am J Clin Pathol 2004;121:S97-104. 
|
[Figure 1]
[Table 1], [Table 2], [Table 3]
| This article has been cited by | | 1 |
Haptoglobin phenotypes as a risk factor for coronary artery disease in type 2 diabetes mellitus: An Egyptian study |
|
| Gehan Hamdy,Olfat M. Hendy,Hala Mahmoud,Azza El-sebaey,Salwa R. Ali,Fatma A. Khalaf | | Egyptian Journal of Medical Human Genetics. 2014; | | [Pubmed] | [DOI] | |
|
 |
|






