|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2013 | Volume
: 19
| Issue : 3 | Page : 293-300 |
| |
Polymorphism at P21 codon 31 and dinucleotide polymorphism of P73 gene and susceptibility to bladder cancer in individuals from North India
Praveen Kumar Jaiswal, Vibha Singh, Rama Devi Mittal
Department of Urology and Renal Transplantation, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India
| Date of Web Publication | 30-Oct-2013 |
Correspondence Address:
Rama Devi Mittal
Department of Urology and Renal Transplantation Sanjay Gandhi Post Graduate Institute of Medical Sciences, Raebareli Road, Lucknow - 226 014, Uttar Pradesh
India
 Source of Support: None, Conflict of Interest: None  | 2 |
DOI: 10.4103/0971-6866.120815

 Abstract Abstract | | |
Background and Aim: p73, a novel P53 homolog and plays an important role in modulating cell cycle control, apoptosis and cell growth while P21, functions to negatively control the cell cycle. P53 up regulates p21 expression in response to deoxyribonucleic acid damage leading to cell cycle arrest at G1 checkpoint. In the present study, we are targeting p21 codon 31 and p73 gene variants of G4C14-to-A4T14 (Exon 2) polymorphism for bladder cancer (BC) risk in North Indians.
Materials and Methods: The above gene variants of P21 and P73 were assessed in the case-control study comprising of 200 BC cases and 200 healthy controls of the same age, gender and similar ethnicity. Genotyping was performed by polymerase chain reaction (PCR) restriction fragment length polymorphism method and PCR-based confronting two-pair primers (PCR with CTPP).
Results: The variant genotype of p73Exon 2 polymorphism showed significant risk for BC (p = 0.014). While combining with heterozygous genotype, variant genotype of p73Exon2 showed a significant association with BC risk (p = 0.010). While in case of p21 codon31 showed no significant association for BC risk at genotypic level. Significant association between p73Exon2 polymorphism and smoking was observed for BC risk. Furthermore, gene combination analysis revealed that AT/AT-Ser/Ser is associated with risk for BC. Variant genotype of P73Exon2 was associated with reduced risk of recurrence (p = 0.039) in superficial BC patients receiving Bacillus Calmette-Guerin treatment thus showing least survival (log rank = 0.029).
Conclusion: Our study provided evidence that the p73 G4C14 > A4T14 (Exon2) polymorphisms were associated with higher risk of BC in North Indian population.
Keywords: Bacillus Calmette-Guerin immunotherapy, gene-gene interaction, P73 and P21 gene polymorphism, survival analysis
How to cite this article:
Jaiswal PK, Singh V, Mittal RD. Polymorphism at P21 codon 31 and dinucleotide polymorphism of P73 gene and susceptibility to bladder cancer in individuals from North India. Indian J Hum Genet 2013;19:293-300 |
How to cite this URL:
Jaiswal PK, Singh V, Mittal RD. Polymorphism at P21 codon 31 and dinucleotide polymorphism of P73 gene and susceptibility to bladder cancer in individuals from North India. Indian J Hum Genet [serial online] 2013 [cited 2016 May 24];19:293-300. Available from: http://www.ijhg.com/text.asp?2013/19/3/293/120815 |
 Introduction Introduction | |  |
Bladder cancer (BC) is an increasingly important international public health problem. BC is nearly 3-4 times more common in men than in women. [1] The incidence rate of BC, which varies among ethnic groups and geographic region, has increased dramatically in recent years. The incidence in India is comparatively low and it is 3.2 in males and 0.7 in females/100,000 people. [2],[3] Risk factors for the development of BC can be classified into three subsets; genetic and molecular abnormalities, chemical or environmental exposures and chronic irritation. [4]
The process of tumor formation and regulation involves a complex combination of genetic, environmental and life-style factors. Complex diseases such as cancer, including BC, have been hypothesized to arise due to the effect of many low-risk gene variants that collectively increase disease risk. [3] Single nucleotide polymorphisms (SNPs) are the most common sequence variations in the human genome and they involve only a single base mutation and can affect coding sequences, splicing and transcription regulation. SNPs can comprehensively reflect genomic hereditary and variation with a large quantity, high density, wide distribution and typical representation. Therefore, SNPs may play increasingly important roles in screening for the gene mutations and susceptibility to oncogenic factors. [5],[6]
The p73 protein is a P53 homolog encoded by a polymorphic gene located on 1p36-33, mapping on a region often deleted in a prostate and lung cancer. [7],[8] p73 protein activates several P53-responsive genes involved in cell cycle control, deoxyribonucleic acid (DNA) repair and apoptosis and inhibits cell growth in a P53-like manner by inducing apoptosis. [9] Extensive molecular analyses of p73 in various tumors suggested that mutations in this gene are rare because they occur in <2% of all cancers. Furthermore, increased p73 expression was found in human malignancies associated with p53 mutations, suggesting p73 may act as a tumor suppressor with some of the same functions as p53 or may compensate the loss of p53 function. [10] It is unknown whether the alteration of p73 expression has any genetic basis such as sequence variations or polymorphisms. At least 17 single-nucleotide polymorphisms have been identified in p73 (some in Exons and others in introns), but none cause an amino acid change. However, two linked single-nucleotide polymorphisms at p73 positions 4 (G > A) and 14 (C > T) are thought to affect p73 function by altering gene expression, perhaps by altering the efficiency of translational initiation.
P21 (CDKN1A), a key cell cycle regulatory protein that governs cell cycle progression from G1 to S phase, can regulate cell proliferation, growth arrest and apoptosis. The Ser31Arg polymorphism also known as p21 codon31 is located in the highly conserved region of p21 and may encode functionally distinct proteins. It is encoded by the CDKN1A gene located on chromosome 6 (6p21.2) and has a p53 transcriptional regulatory motif Thus, most reports focus on genetic variants of p21 and genotypes of some functional polymorphisms have shown to be associated with a high-risk of different types of cancer such as ovarian cancer and breast cancer. [11],[12] Alteration in this gene may adversely affect the regulation of cellular proliferation and increase the susceptibility for cancer. P21WAF1/CIP1 have been speculated to change its function and the high-risk genotypes have been reported to be associated with different cancers including carcinomas of the breast, ovary, [11],[12] cervical cancer [13] and prostate carcinoma. [14]
The most frequently investigated polymorphism of p21 is Ser31Arg (rs1801270C > A), with a base change from C to A resulting in a non-synonymous serine to arginine substitution in the protein [15] causing a loss of the BlPl restriction site and affecting the DNA binding zinc finger motif. [16] Hence, it is likely that Ser31Arg polymorphism may result in the alteration of expression and/or activity, thereby affecting susceptibility to cancer.
Many molecular epidemiological studies have been conducted to evaluate the effect of the Ser31Arg polymorphism on cancer risk. The results, however, remain conflicting and the underlying heterogeneity between studies still needs to be explored.
Given the major roles that p21 codon 31 and p73 G4C14-to-A4T14 Exon 2 play in cell cycle checkpoint regulation, we hypothesized that polymorphisms in the two genes may modify individual susceptibility to BC. Therefore, we evaluated the association between SNPs of p21 and p73 and BC susceptibility in a hospital-based case-control study in North Indian population.
 Materials and Methods Materials and Methods | |  |
Study subjects
The BC patients in this analysis were enrolled from an on-going case-control study of BC, which started patient recruitment in 2010. All enrolled patients were incident cases of histologically confirmed invasive or superficial BC and were recruited from the Urology Department at Sanjay Gandhi Postgraduate Institute of Medical Sciences, a Tertiary Care Center. A total of 200 patients with histological confirmed transitional urothelial BC (mean age 58.5 years; 175 men and 25 women) were recruited for the study. Those with a previous history of other cancer, cancer metastasized to the bladder from another origin and previous radiotherapy was excluded. Healthy and genetically unrelated individuals visiting the hospital for a routine checkup or health awareness camps and hospital employees were recruited as the controls (n = 200). All the controls were age and sex matched with similar ethnicity and had no evidence of malignancy or chronic disease. The mean age of the controls was 56.8 years and M:F ratio as 179:21. The disproportionate ratio between male and female BC in our population could be largely due to increased prevalence in case of males (3:1). The participation rate was 100% and blood samples were available for all subjects. Written informed consent was obtained from all patients and control subjects included in this study. The demographic data was collected using a structured questionnaire, which contained the details of age, stage, disease history, family history and other relevant details such as smoking history, occupation history and other lifestyle factors. Informed and written consent was taken from all subjects when interviewing for the demographic details and blood sample collection. The Ethical Review Board of the Institute approved the study.
Epidemiology data collection
The demographic details were obtained by interviewing each individual in cases and controls. The response rate for the interview was 75% for the subjects. Individuals who smoked once a day for more than 5 years were defined as smokers. The individuals who had never smoked in their lifetime were regarded as non-smokers. At the conclusion of the interview, a 5 ml of a blood sample was drawn into coded vials.
Clinical data collection
The demographic and clinical characteristics of the patients are presented in [Table 1]. The clinical information about tumor size, number, stage and tumor grade, intravesical therapy and dates of recurrence, chemotherapy, radical cystectomy and pathological findings at cystectomy were provided by the Uro-Oncologist in our department. The tumor stages were classified as per American Joint Committee on Cancer's tumour node metastasis staging system. [17] Of the 200 total patients enrolled in the study, 149 patients had non-muscle invasive bladder cancer (NMIBC) while the rest 51 had muscle invasive bladder cancer. Patients with NMIBC at high-risk (high grade, multiple and a large tumor) were treated with intravesical Bacillus Calmette-Guerin (BCG) (n = 78). The patients with non-muscle invasive cancer of low-risk (low grade and single small tumor) were kept on cystoscopic surveillance and considered as Non-BCG patients. Subsequently, all the patients were examined by cystoscopy after every 3 months in the first and second years and later at 6 monthly intervals as long as there was no tumor recurrence. BCG treatment consisted of 6 weekly instillation induction BCG (n = 78). Since the number of patients receiving maintenance BCG was too low, we did not categorize the patients according to BCG regime for statistical analysis. The end point of study included tumor recurrence, defined as a newly found bladder tumor following a previous negative follow-up cystoscopy or end of study time (60 months). Patients with invasive BC (n = 51) were treated with radical cystectomy with or without adjuvant chemotherapy, which included cisplatin, gemcitabine followed-by periodical cystoscopy. Blood sample was collected in ethylene diamine tetra acetate from all subjects for genotyping at the time of enrollment and stored at −70°C.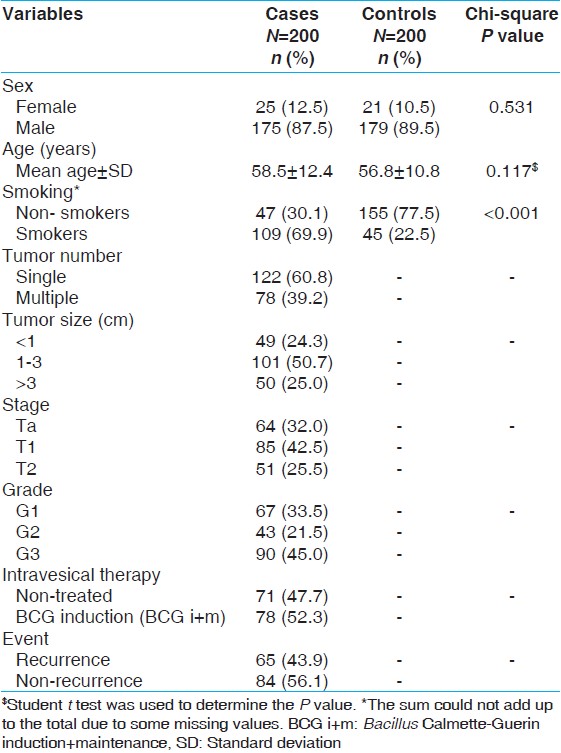 | Table 1: Demographical details of bladder cancer patients and healthy controls
Click here to view |
Genotyping
Genomic DNA was extracted from peripheral blood lymphocytes by salting out method. [18] Polymorphisms in P21 codon31 were analyzed using polymerase chain reaction (PCR) - restriction fragment length polymorphism. The full detail of the primers and PCR conditions were for p21 codon 31,[13] whereas PCR-based confronting two-pair primers (CTPP) (PCR with CTPP) genotyping was performed for the p73 G4C14-to-A4T14 (GC/AT) Exon 2 polymorphism. Primer sequences and PCR conditions used have been described elsewhere [19] and genotyping was done on 2% agarose gel and 10% polyacrylamide gel using molecular weight markers and visualized after staining with ethidium bromide. Positive and negative controls were used in each genotyping assay and 10% of the samples were randomly selected and run in duplicates with 100% concordance. The results were reproducible with no discrepancy in genotyping.
Statistical analysis
The power of the study was calculated using Quanto software, version 1.0 (Freely available from: http://hydra.usc.edu/gxe ) with input of following variables: Case-control study design, significance level (alpha) >0.05 (2 sided), model of inheritance was log additive, allele frequency was 0.28 and the genetic effect for odds ratio (OR) was 1.65 or greater. The present study achieved 80% of the statistical power. The goodness-of-fit Chi-square test was used to analyze any deviation from the Hardy-Weinberg equilibrium in controls. A binary logistic regression model was used to estimate the risk as the OR at the 95% confidence interval (CI). Bonferroni's correction was applied in case of multiple comparisons using the formula p c = p × n (P c - represents corrected value where n is the number of comparisons performed). The statistical analysis was done using the Statistical Package for Social Sciences software, version 15.0 (SPSS, Chicago, IL) and P < 0.05 was considered statistically significant.
 Results Results | |  |
Characteristics of subjects
A total of 200 controls and 200 cases were recruited for this study. There was no significant age difference between the cases (58.5 ± 12.4 years) and the controls (56. 8 ± 10.8 years) (P = 0.117). The cases had significantly higher percentage of smokers (69.9%) than the controls (22.5%) (P = 0.001). The demographic details of the study subjects and clinical characteristics of the patients are presented in [Table 1].
Genotypic frequency of p73Exon2 gene and p21 codon 31 gene polymorphisms in BC
The genotype and allele frequencies of p73Exon2 and p21 codon 31 gene polymorphism in healthy individuals (controls) and BC patients are presented in [Table 2]. The genotype frequencies of controls were in Hardy Weinberg equilibrium. We observed a significant association in the variant genotype of p73Exon2 polymorphism (P = 0.014 OR = 3.80, 95% CI, 1.31-11.01) with BC risk, whereas no association with p21 codon 31 was observed. However, combined heterozygous and variant genotype showed risk for BC (P = 0.010 OR = 1.58, 95% CI, 1.12-2.23).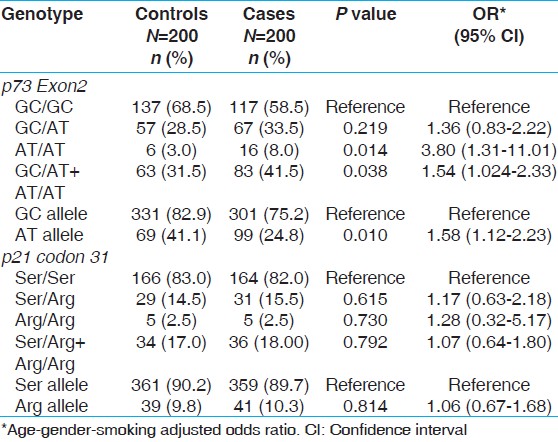 | Table 2: Genotypic frequency of p73Exon2 and p21 codon 31 gene polymorphisms in bladder cancer and healthy controls
Click here to view |
Association of p73Exon2 gene and p21 codon 31 genotypes with smoking
We evaluated the gene smoking interaction to study the modulation of BC risk with respect to p73 and p21 codon 31. The patients were grouped as non-smokers and smokers. In case of p73 G4C14 > A4T14 gene, the hetero genotype GC/AT was associated with higher risk of BC (P = 0.017; OR, 3.02; 95% CI, 1.22-7.46). However, no significant association was observed in case of p21 codon 31 [Table 3].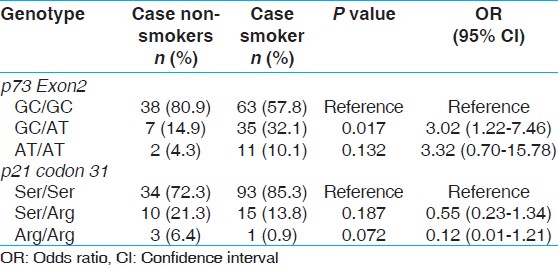 | Table 3: Influence of smoking in bladder cancer patients smokers versus bladder cancer patients non-smokers
Click here to view |
Association of p73Exon2 gene and p21 codon 31 genotypes with tumor stage/grade
The patients with similar stage but with different grades respond to treatment differently. Hence we stratified the patients into three sub-groups according to stage/grade (TaG1 [low-risk NMIBC], TaG 2,3 + T1G 1-3 [High-risk NMIBC] and T2+ [muscle invasive]). TaG 1 was taken as a reference. A reduced association was observed in case of p73 G4C14 > A4T14 gene polymorphism combined genotype GC/AT + AT/AT showed a higher risk for BC when TaG1 and TaG 2,3 + T1G 1-3 was considered for calculation (P = 0.001; OR 0.27; 95% CI, 0.12-0.58) while in case of TaG1 and T2 + stage tumor also showed reduced risk for BC (P = 0.046, OR, 0.41; 95% CI, 0.17-0.98) [Table 4].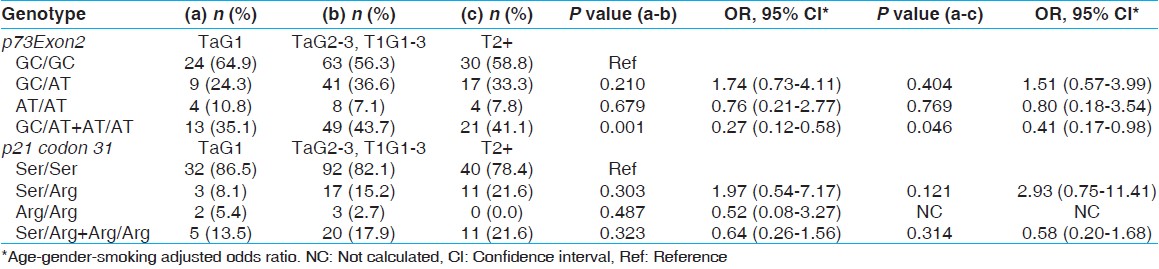 | Table 4: Influence of p73Exon2 and p21 codon 31 gene polymorphisms on the tumor-stage/grade in BC patients
Click here to view |
Modulation of genotype variants and outcome after BCG immunotherapy
To analyze the association of gene polymorphisms of p73Exon2 and p21 codon 31 and risk of recurrence in NMIBC patients, further analysis was restricted to NMIBC patients only (n = 149). The median follow-up of NMIBC patients was 14 months (3-60 months). We analyzed the association of genotypes and risk of recurrence after BCG immunotherapy. We grouped patients into BCG treated (n = 78) and non-treated (n = 71) as these were patients of low grade tumors and did not require BCG immunotherapy. The patients of "BCG group" with variant genotype (AT/AT) of p73 G4C14 > A4T14 were observed to have high-risk of recurrence (p = 0.039; HR, 3.04) [Table 5]. 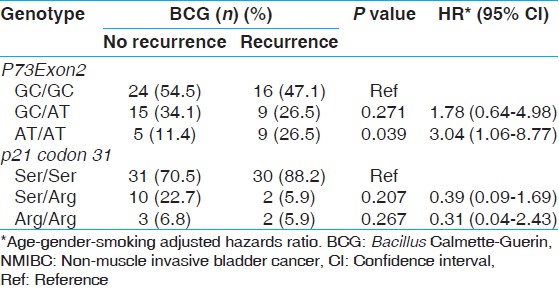 | Table 5: Influence of P73Exon2 and p21 codon 31 gene polymorphisms on the outcome of BCG treated NMIBC patients and risk of recurrence in bladder cancer
Click here to view |
Subsequently, Kaplan-Meier analysis of p73 G4C14 > A4T14 showed a higher recurrence free survival 46 months of GC/GC genotype as compared with 38 months of GG/AT genotype carrying patients While variant genotype AT/AT showed least survival with 25 months (log rank P = 0.029) [Figure 1]. p21 codon 31 gene polymorphism were not associated with risk of recurrence free survival.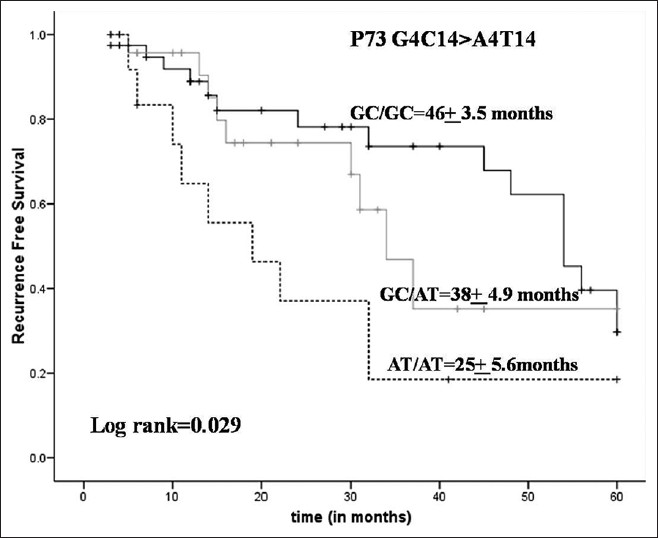 | Figure 1: Kaplan Meir survival analysis for Bacillus Calmette-Guerin treated bladder cancer patient in relation to P73 G4C14 > A4T14 (Median recurrence free survival for GC/GC = 46 months, GC/AT = 38 months, and AT/AT = 25 months; log rank = 0.029)
Click here to view |
Gene combination effect between p73 and P21 polymorphism gene polymorphism
To analyze the combined effect of these polymorphisms, we conducted gene-gene interaction analysis. Gene combination between p73 and p21 showed a significant risk of BC (AT/AT-Ser/Ser p = 0.022; OR, 3.43; 95% CI; 1.19-9.86) with 3.43 fold risk [Table 6].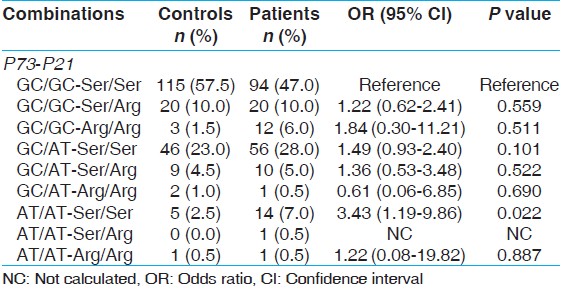 | Table 6: Gene combination effect of P73 and P21 polymorphism in bladder cancer patients and healthy controls
Click here to view |
 Discussion Discussion | |  |
The p53 gene and its encoded protein are related with the regulation of cell cycle, cellular growth and apoptosis. Recent studies have revealed that several genetic polymorphisms may play important roles in the pathogenesis of BC such as Survivin - 31G/C [20] and CCL2 I/D [21] in north Indian population. In this hospital-based case-control study, we found that the variant genotypes (GC/AT + AT/AT) and variant allele of p73 were associated with an increased risk of BC. Although how this p73 polymorphism influences the development of cancer is unknown, one possible explanation is that the GC to AT change may lead to the formation of a stem-loop structure and so may influence the translation efficiency of p73. [22] Another possibility is that the p73 polymorphism is in linkage disequilibrium with other functional polymorphisms that affect either the expression or activity levels of enzymes involved in tumorigenesis. [23] However, a study in breast cancer showed contrary result that the GC/GC genotype of P73Exon2 is significantly associated with poor prognosis in breast cancer. [24] In another study indicated that P73Exon2 AT/AT homozygotes appear to be protected in oesophageal cancer. [25] Consistent with these contradictory results, several studies have correlated with an increased risk of various cancers, including the head and neck cancer [19],[26] and lung cancer. [27] Our study showed no significant association of p21 codon 31 gene polymorphism with BC risk. Although recent studies suggest that the p21 polymorphism is likely to contribute to genetic susceptibility to cancer in case of Lung carcinoma. [28] Some studies including ours found no association between the polymorphism and cancer risk along with study in lung cancer. [29] However, our observation was in line with some of study nasopharyngeal carcinoma [30] and with esophageal squamous cell carcinoma showing no association with disease. [31],[32]
This discrepancy could be due to different carcinogenic mechanisms among different cancer types, as well as differences in the underlying genetic backgrounds and/or environmental and social factors in different populations studied. A study in Korean population showed that codon 31 Ser/Se homozygote of the p21 gene could be a risk factor for the development of cervical adenocarcinoma associated with high-risk human papillomavirus. [13] The same result was found in case of esophageal cancer showing p21 codon 31 Ser homozygosity conferred risk for the process of developing esophageal cancer. [32] However, there were contradictory result in case of BC an individual possessing one allele of arginine form in p21 codon 31 has a higher risk of developing BC than the serine form. [33] Arg/Arg genotype was found to have an increased risk of developing prostate cancer and Benign Prostatic Hyperplasia compared with those having the Ser/Ser genotype. [34]
We also analyzed gene-gene combination to evaluate the synergistic effect on BC. A significant association with combining p73 and p21 codon31, AT/AT-Ser/Ser showed significant association with BC.
The data of our study provided evidence for the association of p73 G4C14 > A4T14 polymorphisms and risk of recurrence in NMIBC patients in our population. AT/AT mutant genotype was associated with high-risk of recurrence (HR = 3.04, P = 0.039) as compared with wild-type genotype (GC/GC), in superficial BC patients receiving BCG treatment. The Kaplan-Meier curve further enumerates the potential association of GC/GC genotype to be associated with increased recurrence-free survival with a median of 46 months (P = 0.029). This suggests that GC genotype is associated with good clinical outcome in NMIBC patients who were on BCG treatment.
The genetic variations that might be stabilized among the population are those that cause no pivotal effect in normal condition. However, under special circumstances the variations may predispose the carriers to diseases. Therefore, analysis combined with the genetic variations and the other internal and external factors may lead to a better understanding of the role of genetic makeup in cancer pathogenesis.
This analysis suggests future work in subsequent directions such as working with large samples and detailed surveys focusing on the functional pattern of this polymorphism, examining p73 and p21 expression levels by genotype among the current population, analysis of genotypic interactions with closely related genes. Further research of this critical gene and those which are biologically related may lead to a better biological understanding of BCs. Validating its independent prognostic value for clinical diagnosis and the outcome is of great implication. In addition, findings such as these will lead to the development of genetic risk prediction panels for eventual classification of patients who may most benefit from targeted surveillance or prevention strategies.
 Acknowledgments Acknowledgments | |  |
We are thankful to the Urologists in our department for providing samples and relevant clinical information of patients. PKJ is thankful to UGC for providing Junior Research Fellowship and VS is thankful to ICMR for providing Senior Research Fellowship.
 References References | |  |
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. 
|
| 2. | Shariat SF, Sfakianos JP, Droller MJ, Karakiewicz PI, Meryn S, Bochner BH. The effect of age and gender on bladder cancer: A critical review of the literature. BJU Int 2010;105:300-8. 
|
| 3. | Sinha R, Anderson DE, McDonald SS, Greenwald P. Cancer risk and diet in India. J Postgrad Med 2003;49:222-8. 
[PUBMED]  |
| 4. | Franekova M, Halasova E, Bukovska E, Luptak J, Dobrota D. Gene polymorphisms in bladder cancer. Urol Oncol 2008;26:1-8. 
|
| 5. | Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science 1996;273:1516-7. 
|
| 6. | Erichsen HC, Chanock SJ. SNPs in cancer research and treatment. Br J Cancer 2004;90:747-51. 
|
| 7. | Takahashi H, Ichimiya S, Nimura Y, Watanabe M, Furusato M, Wakui S, et al. Mutation, allelotyping, and transcription analyses of the p73 gene in prostatic carcinoma. Cancer Res 1998;58:2076-7. 
|
| 8. | Nomoto S, Haruki N, Kondo M, Konishi H, Takahashi T, Takahashi T, et al. Search for mutations and examination of allelic expression imbalance of the p73 gene at 1p36.33 in human lung cancers. Cancer Res 1998;58:1380-3. 
|
| 9. | Schabath MB, Wu X, Wei Q, Li G, Gu J, Spitz MR. Combined effects of the p53 and p73 polymorphisms on lung cancer risk. Cancer Epidemiol Biomarkers Prev 2006;15:158-61. 
|
| 10. | Choi HR, Batsakis JG, Zhan F, Sturgis E, Luna MA, El-Naggar AK. Differential expression of p53 gene family members p63 and p73 in head and neck squamous tumorigenesis. Hum Pathol 2002;33:158-64. 
|
| 11. | Goode EL, Fridley BL, Vierkant RA, Cunningham JM, Phelan CM, Anderson S, et al. Candidate gene analysis using imputed genotypes: Cell cycle single-nucleotide polymorphisms and ovarian cancer risk. Cancer Epidemiol Biomarkers Prev 2009;18:935-44. 
|
| 12. | Driver KE, Song H, Lesueur F, Ahmed S, Barbosa-Morais NL, Tyrer JP, et al. Association of single-nucleotide polymorphisms in the cell cycle genes with breast cancer in the British population. Carcinogenesis 2008;29:333-41. 
|
| 13. | Roh J, Kim M, Kim J, Park N, Song Y, Kang S, et al. Polymorphisms in codon 31 of p21 and cervical cancer susceptibility in Korean women. Cancer Lett 2001;165:59-62. 
|
| 14. | Kibel AS, Suarez BK, Belani J, Oh J, Webster R, Brophy-Ebbers M, et al. CDKN1A and CDKN1B polymorphisms and risk of advanced prostate carcinoma. Cancer Res 2003;63:2033-6. 
|
| 15. | Chedid M, Michieli P, Lengel C, Huppi K, Givol D. A single nucleotide substitution at codon 31 (Ser/Arg) defines a polymorphism in a highly conserved region of the p53-inducible gene WAF1/CIP1. Oncogene 1994;9:3021-4. 
|
| 16. | Mousses S, Ozçelik H, Lee PD, Malkin D, Bull SB, Andrulis IL. Two variants of the CIP1/WAF1 gene occur together and are associated with human cancer. Hum Mol Genet 1995;4:1089-92. 
|
| 17. | Colombel M, Soloway M, Akaza H, Böhle A, Palou J, Buckley R, et al. Epidemiology, staging, grading, and risk stratification of bladder cancer. Eur Urol Suppl 2008;7:618-26. 
|
| 18. | Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988;16:1215. 
|
| 19. | Li G, Sturgis EM, Wang LE, Chamberlain RM, Amos CI, Spitz MR, et al. Association of a p73 exon 2 G4C14-to-A4T14 polymorphism with risk of squamous cell carcinoma of the head and neck. Carcinogenesis 2004;25:1911-6. 
|
| 20. | Jaiswal PK, Goel A, Mandhani A, Mittal RD. Functional polymorphisms in promoter survivin gene and its association with susceptibility to bladder cancer in North Indian cohort. Mol Biol Rep 2012;39:5615-21. 
|
| 21. | Singh V, Srivastava P, Srivastava N, Kapoor R, Mittal RD. Association of inflammatory chemokine gene CCL2I/D with bladder cancer risk in North Indian population. Mol Biol Rep 2012;39:9827-34. 
|
| 22. | Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell 1997;90:809-19. 
|
| 23. | Haber DA, Fearon ER. The promise of cancer genetics. Lancet 1998;351 Suppl 2:SII1-8. 
|
| 24. | Li H, Yao L, Ouyang T, Li J, Wang T, Fan Z, et al. Association of p73 G4C14-to-A4T14 (GC/AT) polymorphism with breast cancer survival. Carcinogenesis 2007;28:372-7. 
|
| 25. | Ryan BM, McManus R, Daly JS, Carton E, Keeling PW, Reynolds JV, et al. A common p73 polymorphism is associated with a reduced incidence of oesophageal carcinoma. Br J Cancer 2001;85:1499-503. 
|
| 26. | Gallì P, Cadoni G, Volante M, De Feo E, Amore R, Giorgio A, et al. A case-control study on the combined effects of p53 and p73 polymorphisms on head and neck cancer risk in an Italian population. BMC Cancer 2009;9:137. 
|
| 27. | Li G, Wang LE, Chamberlain RM, Amos CI, Spitz MR, Wei Q. p73 G4C14-to-A4T14 polymorphism and risk of lung cancer. Cancer Res 2004;64:6863-6. 
|
| 28. | Popanda O, Edler L, Waas P, Schattenberg T, Butkiewicz D, Muley T, et al. Elevated risk of squamous-cell carcinoma of the lung in heavy smokers carrying the variant alleles of the TP53 Arg72Pro and p21 Ser31Arg polymorphisms. Lung Cancer 2007;55:25-34. 
|
| 29. | Su L, Liu G, Zhou W, Xu LL, Miller DP, Park S, et al. No association between the p21 codon 31 serine-arginine polymorphism and lung cancer risk. Cancer Epidemiol Biomarkers Prev 2003;12:174-5. 
|
| 30. | Tsai MH, Chen WC, Tsai FJ. Correlation of p21 gene codon 31 polymorphism and TNF-alpha gene polymorphism with nasopharyngeal carcinoma. J Clin Lab Anal 2002;16:146-50. 
|
| 31. | Taghavi N, Biramijamal F, Abbaszadegan MR, Khademi H, Sotoudeh M, Khoshbakht S. P21(waf1/cip1) gene polymorphisms and possible interaction with cigarette smoking in esophageal squamous cell carcinoma in northeastern Iran: A preliminary study. Arch Iran Med 2010;13:235-42. 
|
| 32. | Yang W, Qi Q, Zhang H, Xu W, Chen Z, Wang L, et al. p21 Waf1/Cip1 polymorphisms and risk of esophageal cancer. Ann Surg Oncol 2010;17:1453-8. 
|
| 33. | Chen WC, Wu HC, Hsu CD, Chen HY, Tsai FJ. p21 gene codon 31 polymorphism is associated with bladder cancer. Urol Oncol 2002;7:63-6. 
|
| 34. | Huang SP, Wu WJ, Chang WS, Wu MT, Chen YY, Chen YJ, et al. p53 Codon 72 and p21 codon 31 polymorphisms in prostate cancer. Cancer Epidemiol Biomarkers Prev 2004;13:2217-24. 
|
[Figure 1]
[Table 1], [Table 2], [Table 3], [Table 4], [Table 5], [Table 6]
|






