|
 
 |
| CASE REPORT |
|
| Year : 2014 | Volume
: 2
| Issue : 1 | Page : 30-33 |
|
Amyotrophic lateral sclerosis as sole neurological manifestation of human immunodeficiency virus
Subrata Chakrabarti, Koushik Pan, Kapil Mondal, Sujoy Panchadhyayee
Department of General Medicine, IPGMER, Kolkata, West Bengal, India
| Date of Web Publication | 1-Jul-2014 |
Correspondence Address:
Subrata Chakrabarti
Room No. 414, Doctor's Hostel, IPGMER, AJC Bose Road, Kolkata 700 020, West Bengal
India
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/2321-9157 .135746

Motor neuron diseases (MND) are extremely rare neurological manifestations of human immunodeficiency virus (HIV). We report a case of a 33-year-old known HIV seropositive male who presented with progressive asymmetrical onset of weakness and wasting of both distal limbs along with dysphagia and difficulty in speech. Examination revealed significant atrophy with visible fasciculations in thenar and hypothenar areas of both hands and dorsum of both feet associated with brisk deep tendon reflexes and jaw jerks, but diminished gag reflexes. Electromyography revealed evidence of denervation pattern. Investigations for underlying causes of MND other than HIV were noncontributory. Administration of riluzole along with continuation of antiretroviral therapy brought significant relief to his limb weakness, but bulbar features continued to progress. The case report highlights the rare, but definite association between HIV and amyotrophic lateral sclerosis (ALS) and partial reversibility of disabling clinical features on highly active antiretroviral therapy, which mandates ruling out HIV in all cases of ALS. Keywords: Amyotrophic lateral sclerosis, electromyography, human immunodeficiency virus, motor neuron diseases
How to cite this article:
Chakrabarti S, Pan K, Mondal K, Panchadhyayee S. Amyotrophic lateral sclerosis as sole neurological manifestation of human immunodeficiency virus. J HIV Hum Reprod 2014;2:30-3 |
How to cite this URL:
Chakrabarti S, Pan K, Mondal K, Panchadhyayee S. Amyotrophic lateral sclerosis as sole neurological manifestation of human immunodeficiency virus. J HIV Hum Reprod [serial online] 2014 [cited 2018 Feb 28];2:30-3. Available from: http://www.j-hhr.org/text.asp?2014/2/1/30/135746 |
| Introduction | |  |
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive, ultimately fatal neurodegenerative disorder for which there is no effective treatment. The diagnosis is based upon the combination of clinical presentation and electro-diagnostic studies. In general, there is a mixture of upper and lower motor neuron signs. Clinical and electro-diagnostic studies are almost confirmative of ALS. [1] Magnetic resonance imaging (MRI) brain often help in corroborating the diagnosis in appropriate clinical settings. Both studies and case reports have documented a definite association between this neurodegenerative disorder and human immunodeficiency virus (HIV). We report such a case of unique association.
| Case report | |  |
A 33-year-old heterosexual, nondiabetic, nonhypertensive male, who was also a tobacco-smoker and intravenous drug abuser (heroin abuser) presented with progressive asymmetric distal muscle weakness along with thinning and spontaneous twitching movements in both upper and lower limbs for last 2 months. He also complained of progressive dysphagia, which was more toward liquids than solids. He also suffered from recurrent choking episodes during taking foods. He was also troubled by progressive difficulty in speech and hoarseness. His dysphagia and hoarseness had no diurnal variability or fatigability. No history of any diplopia or dribbling of saliva from angle of mouth. Furthermore, no history of any bladder-bowel or cortical or meningeal or sensory involvement was elicited. He was diagnosed HIV-1 seropositive (subtype C) 7 years back, and was on grossly irregular antiretroviral therapy (zidovudine, lamivudine, and saquinavir) for last 3 years. Patient was on this second line therapy after discontinuation of his first line highly active antiretroviral therapy (HAART) regimen of zidovudine, lamivudine, and nevirapine 3 years back due to finding of progressively increasing viral ribonucleic acid titers suggestive of regimen failure and demonstration of high grade resistance to nevirapine during an episode of cryptococcal meningitis. Large pill burden of saquinavir combined with lack of family and emotional support led him to take drugs irregularly. There was no past history of suffering from or having been vaccinated against, poliomyelitis. There was no family history of neurological disease. On general examination, significant pallor was noted, but there was no lymphadenopathy. Neurological examination showed he was alert and orientated; spastic dysarthria and marked fasciculation in his atrophic tongue were noted. Gag reflexes were diminished bilaterally. Other cranial nerves were intact. In muscle testing, there was significant atrophy in thenar and hypothenar areas of both hands [Figure 1] and dorsum of both feet [Figure 2] without involvement of trunk or extraocular muscles. Fasciculations were noted in the distal wasted muscles. Muscle power testing showed power of distal muscles of both upper and lower limbs as 2/5 and proximally 4/5 according to Medical Research Council grading. Neck flexion was weak. Jaw jerk and tendon reflexes were brisk bilaterally. Plantar response was extensor bilaterally. No other abnormality in neurological examination (including cerebellum and sensory modalities) was noted. Other system examination revealed no abnormality. With mixed upper and lower motor neuron deficits in all limbs and in bulbar territory, a diagnosis of clinically definite ALS (El Escorial) was made.
The CD4 count was 230 cells × 10 6 /l and viral load was 127,000 copies/ml. An electromyogram showed generalized fibrillation and positive sharp waves suggestive of widespread denervation pattern in all muscle groups tested in both proximal and distal limbs, both upper and lower limbs. This pattern was classical of motor neuron disease (MND). Nerve conduction study showed diminished compound motor action potential with normal conduction velocity in both the median, ulnar, and peroneal nerves. Sensory motor action potential, F-wave, and H-reflex were normal. There was no evidence of multifocal motor neuropathy with conduction block. Tests were performed in order to exclude secondary causes of a lower motor neuron type of muscle weakness in this patient (other than HIV) whereby normal results were obtained for renal function tests, serum electrolytes including sodium, potassium, calcium, phosphate, liver function tests (except raised globulin level), fasting and postprandial blood glucose, creatine phosphokinase, thyroid function tests, serum protein electrophoresis, serum B12, and red blood cell folate. Peripheral blood picture suggested normocytic, normochromic anemia.
Cerebrospinal fluid (CSF) study was normal except for a marginal protein rise (51 mg/dl; normal 20-40 mg/dl). Syphilis serology by treponema pallidum hemagglutination assay, rheumatoid factor, antinuclear factor (ANF), antinuclear antibody, and ANF profile (including antineuronal nuclear antibody type 1), antiganglioside antibodies, acetylcholine receptor antibodies and human T-cell lymphocytotropic virus type 1 (HTLV-1) antibodies were all negative. Lyme serology was not done as Lyme disease is not common in India and so facilities for testing were not available. Serum lead level was normal. MRI of the corticomedullary junction and cervical spine, with and without contrast, ruled out any compressive myeloradiculopathy. MRI brain showed hyperintensity in bilateral corticospinal tract in the region of internal capsule, which was classical of ALS [Figure 3]. A final diagnosis of ALS secondary to HIV was made. He was administered new HAART regimen comprising of zidovudine, lamivudine, and ritonavir (saquinavir was discontinued due to its high dose requirement, which had led to poor adherence previously). Strict adherence to treatment was ensured after counseling sessions of the patient and education of his family members regarding the importance of regular intake of drugs in this patient. Alongside, he was started on riluzole 50 mg twice a day. Follow-up after 3 months revealed marginal improvement in limb muscle weakness, but no improvement in bulbar function.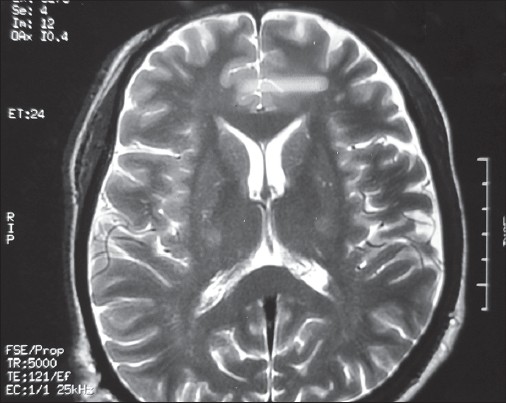 | Figure 3: Hyperintensities in both corticospinal tracts in the region of internal capsule
Click here to view |
| Discussion | |  |
Motor neuron disease is a neurodegenerative disorder, characterized clinically by progressive weakness of limb, bulbar, and respiratory muscles. Clinically, definite MND (El Escorial definite ALS) requires the presence of mixed upper and lower motor neuron deficits in three or more anatomical areas. [1] The cause of sporadic MND remains unknown, although a defect in Cu/Zn superoxide dismutase-1 accounts for about 1% of all cases.
Viral etiology of MND was first hypothesized in 1969 by Poskanzer et al. based on his observation that poliovirus selectively infects motor neurons in the brain stem and spinal cord. [2] It was demonstrated subsequently, that HTLV-1 also can cause a MND syndrome. [3] Evidence of pyramidal tract, anterior horn cell involvement and lymphocytic inflammation was documented at autopsy in cases of HTLV-1 myelopathy. [4]
Reports of HIV associated MND are sparse in the literature. HIV-associated MND was first reported in 1985. Since then, more than 20 case reports have appeared depicting this association. Higher frequency of MND in HIV seropositive population [5] and reports of slowing, [6] arrest, [5] or occasional reversal, [7] of motor deficit following antiretroviral therapy in these cases, have been proposed as evidence for the causal relation between the HIV infection and MND. Sinha et al. in their study have reported the first case of HIV related MND from India. [8] Association between HIV and ALS is not merely coincidental, but etiologically related as suggested by Nishio et al. [6] and Galassi et al. [9] In fact ALS in HIV-positive people may take either of two forms, one that responds to HAART and another that does not. The responsive form seems to be related to viral infection. Harbingers of therapeutic response are young age at onset, progression in days or weeks, and abnormal CSF. The unresponsive form may be "ordinary" sporadic ALS that occurs by chance in an HIV-positive person. [10] In one study by Moulignier et al. conducted over a 13-year period, six patients with HIV-1 infection in a large series of 1700 patients were confirmed to have an ALS-like disorder, that is, a frequency of 3.5/1000, which greatly exceeds the expected incidence of ALS in the general population of 0.4-1.76/100,000. [5] The same study (by Moulignier et al.) pointed out some salient features of HIV associated MND The disorder differed from classical ALS in several respects: Earlier age of onset, rapid progression, and clinical improvement in response to antiretroviral therapy (including complete recovery in two patients. According to them, several possible mechanisms by which HIV might affect motor neurons include neuronal infection, response to toxic viral products, immune induction resulting in cytokine secretion, and autoimmune disease causing acquired defects of superoxide dismutase. Not only that viral quasispecies may play in the pathogenesis, but the presence of defined HIV-1 strains is also necessary in this regard. Every patient with ALS should be tested for HIV-1 (for potential therapeutic option) as well as should be tested for HIV-1 viral quasispecies to identify preferential species (for research interest). [5] Whether HIV itself or unknown accompanying infection is responsible for motor neuron findings or completely unrelated cannot be currently concluded with certainty at present. However, findings of reverse transcriptase, antiretroviral antibodies, supportive animal models suggest strongly that HIV is either causative or contributory. [11]
| Conclusion | |  |
Since clinical improvement in response to HAART, concomitant with suppression of viral load, and increases in CD4 counts, is a definite possibility; in ALS it is recommended that the search for HIV as a likely etiology of ALS should be undertaken vigorously.
| References | |  |
| 1. | Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial "Clinical limits of amyotrophic lateral sclerosis" workshop contributors. J Neurol Sci 1994;124 Suppl: 96-107. 
|
| 2. | Poskanzer DC, Cantor HM, Kaplan GS. The frequency of preceding poliomyelitis in amyotrophic lateral sclerosis. In: Norris FH Jr, Kurland LT, editors. Motor Neuron Disease: Research on Amyotrophic Lateral Sclerosis and Related Disorders. New York: Grune and Stratton; 1969. p. 286-90. 
|
| 3. | Jubelt B. Viruses and motor neuron diseases. Adv Neurol 1991;56:463-72. 
|
| 4. | Hoffman PM, Festoff BW, Giron LT Jr, Hollenbeck LC, Garruto RM, Ruscetti FW. Isolation of LAV/HTLV-III from a patient with amyotrophic lateral sclerosis. N Engl J Med 1985;313:324-5. 
|
| 5. | Moulignier A, Moulonguet A, Pialoux G, Rozenbaum W. Reversible ALS-like disorder in HIV infection. Neurology 2001;57:995-1001. 
|
| 6. | Nishio M, Koizumi K, Moriwaka F, Koike T, Sawada K. Reversal of HIV-associated motor neuron syndrome after highly active antiretroviral therapy. J Neurol 2001;248:233-4. 
|
| 7. | MacGowan DJ, Scelsa SN, Waldron M. An ALS-like syndrome with new HIV infection and complete response to antiretroviral therapy. Neurology 2001;57:1094-7. 
|
| 8. | Sinha S, Mathews T, Arunodaya GR, Siddappa NB, Ranga U, Desai A, et al. HIV-1 clade-C-associated "ALS"- like disorder: First report from India. J Neurol Sci 2004;224:97-100. 
|
| 9. | Galassi G, Gentilini M, Ferrari S, Ficarra G, Zonari P, Mongiardo N, et al. Motor neuron disease and HIV-1 infection in a 30-year-old HIV-positive heroin abuser: A causal relationship? Clin Neuropathol 1998;17:131-5. 
|
| 10. | Rowland LP. HIV-related neuromuscular diseases: Nemaline myopathy, amyotrophic lateral sclerosis and bibrachial amyotrophic diplegia. Acta Myol 2011;30:29-31. 
|
| 11. | Berger JR, Espinosa PS, Kissel J. Brachial amyotrophic diplegia in a patient with human immunodeficiency virus infection: Widening the spectrum of motor neuron diseases occurring with the human immunodeficiency virus. Arch Neurol 2005;62:817-23. 
|
[Figure 1], [Figure 2], [Figure 3]
|