|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2015 | Volume
: 3
| Issue : 2 | Page : 34-40 |
|
Decline in positivity rates among HIV-exposed infants with changes in prevention of mother-to-child transmission antiretroviral regimens in Nigeria: Evidence from 7 years of field implementation
Hadiza Khamofu1, Edward A Oladele1, Uche Ralph-Opara1, Titi Badru1, Oluwasanmi Adedokun1, Mariya Saleh1, McPaul Okoye2, Olufunsho Adebayo3, Kwasi Torpey1
1 FHI 360, Nigeria
2 United States Agency for International Development (USAID), Abuja, Nigeria
3 FHI 360, Pretoria, South Africa
| Date of Web Publication | 14-Jul-2016 |
Correspondence Address:
Edward A Oladele
Department of Prevention, Care and Treatment, 1073, Godab Plaza, FHI 360
Nigeria
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/2321-9157.186351

Objective: Demonstrate if the introduction of more and more efficacious antiretroviral (ARV) combinations for prevention of mother-to-child transmission (PMTCT) over time translated into a declining HIV-infection among HIV-exposed infants. Methods: This was a retrospective review of routinely collected PMTCT service data from 2008 to 2014 in 682 secondary and tertiary health facilities across Nigeria. The ARV regimen was measured by the proportions of different ARV regimens received by HIV-positive pregnant women each year and the HIV-infection among infants was determined by the rate of HIV-positive polymerase chain reaction tests each year. The District Health Information Software (DHIS) was used to extract data from health facilities. The same DHIS was used to aggregate and analyze data. Results: Maternal HIV positivity rates varied from 4.1% in 2008, 2.9% in 2011, and 3.2% in 2012, then declined steadily to 1.9% in 2014. The total number of pregnant women who tested positive for HIV and received different ARV regimen for PMTCT during the period (2008-2014) was 63,774; ranging from 7506 in 2008 to 10,388 in 2014. Uptake of single dose nevirapine by the positive pregnant women was 34.4%, 41.6%, and 45.9% in 2008, 2009, and 2010, respectively. HIV positive pregnant women on triple ARVs (prophylaxis or treatment) increased from 22% in 2008 to 99% in 2014. Infant HIV positivity rates showed a steady decline over the years, from 38% in 2008 to 6% in 2014 (P < 0.001). Conclusions: We demonstrated the declining trend of HIV-infection among HIV-exposed infant in Nigeria as more and more efficacious ARV regimens were available for HIV-positive pregnant women. We conclude that if current efforts were sustained and coverage widened, an alignment of the country's PMTCT program with the best available scientific evidence could lead to the elimination of mother to child transmission. Keywords: Child, early infant diagnosis, healthcare, HIV transmission, Nigeria, pregnancy, retrospective studies
How to cite this article:
Khamofu H, Oladele EA, Ralph-Opara U, Badru T, Adedokun O, Saleh M, Okoye M, Adebayo O, Torpey K. Decline in positivity rates among HIV-exposed infants with changes in prevention of mother-to-child transmission antiretroviral regimens in Nigeria: Evidence from 7 years of field implementation. J HIV Hum Reprod 2015;3:34-40 |
How to cite this URL:
Khamofu H, Oladele EA, Ralph-Opara U, Badru T, Adedokun O, Saleh M, Okoye M, Adebayo O, Torpey K. Decline in positivity rates among HIV-exposed infants with changes in prevention of mother-to-child transmission antiretroviral regimens in Nigeria: Evidence from 7 years of field implementation. J HIV Hum Reprod [serial online] 2015 [cited 2018 Aug 6];3:34-40. Available from: http://www.j-hhr.org/text.asp?2015/3/2/34/186351 |
| Introduction | |  |
The risk of mother to child transmission of HIV (MTCT) in Nigeria is high due to a combination of factors which include; a high fertility rate of 5.5%; [1] HIV prevalence of 4.1% among women attending antenatal clinic (ANC); [2] and low coverage of prevention of mother-to-child transmission (PMTCT) services (30% as at end of 2013). [3] The 51,000 new HIV infections recorded in Nigeria in 2013 accounted for one-quarter of all new HIV infections among children in the 21 Global Plan priority countries. [4],[5] MTCT is responsible for the majority of these HIV infections among children. [6] Without antiretroviral drugs (ARVs), the rate of MTCT is estimated to be about 15-45%, with more than half of the HIV-infected infants dying before the age of 1 year. [7]
Evidence from controlled studies has shown that the use of ARVs and other interventions have the potential to reduce pediatric HIV infections to <2%. [8],[9] This has created the optimism that MTCT can be eliminated. Accordingly, PMTCT programs have provided ARVs to an increasing number of HIV-infected pregnant women. In Nigeria for example, the number of health facilities providing PMTCT services increased from only 6 in 2004 to 6000 in 2014.
As countries scaled up PMTCT services, scientific evidence was also evolving leading to changes in recommended ARVs for PMTCT to more efficacious regimens. Nigeria's PMTCT program has evolved over the years with changes in guideline recommendations, including cut-off for antiretroviral therapy (ART) eligibility and transition from monotherapy (single dose nevirapine [sdNVP]) to the use of more efficacious triple ARVs for PMTCT. [10],[11],[12] These changes are summarized in [Table 1].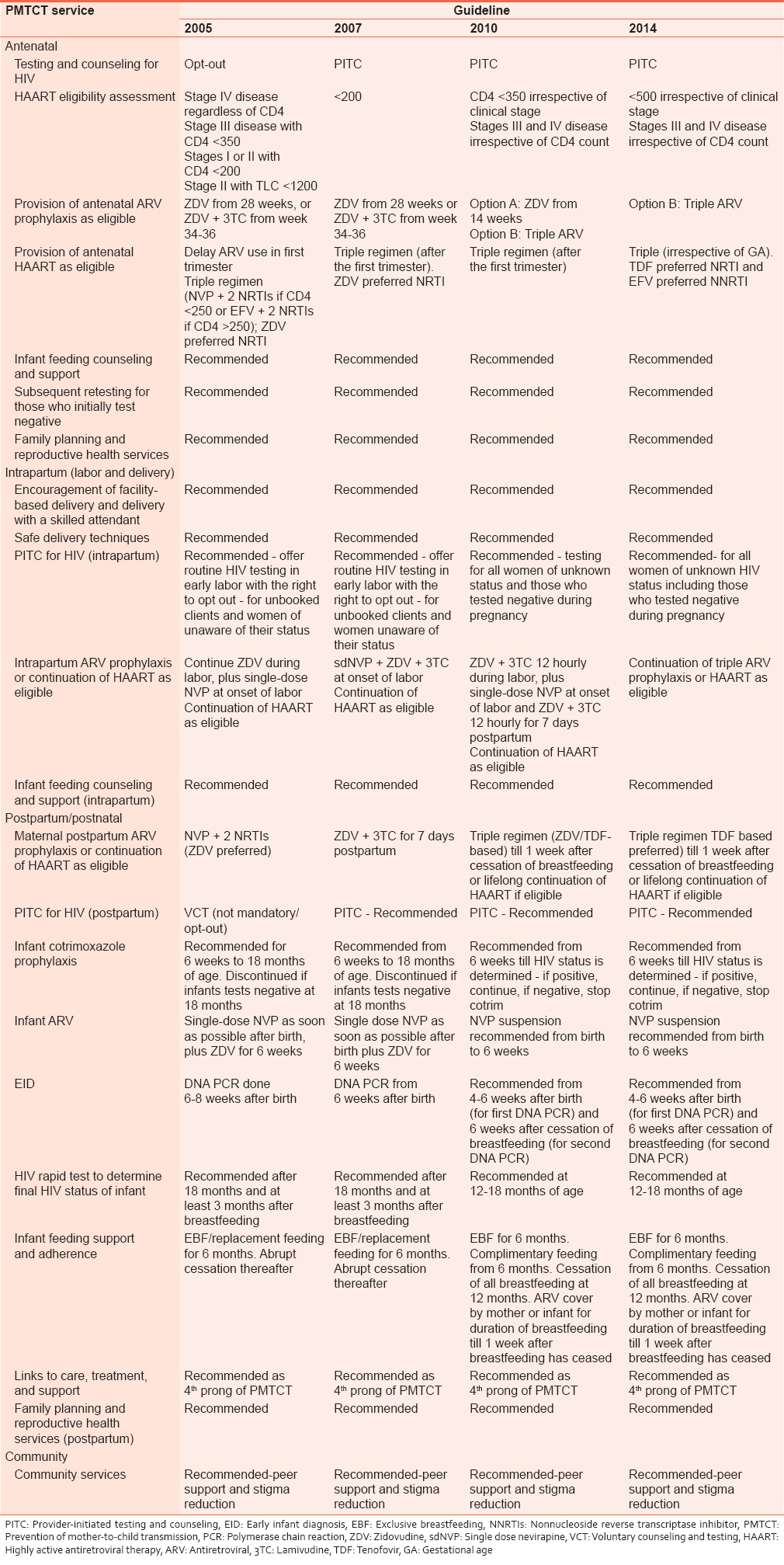 | Table 1: Summary of key changes in national guidelines between 2005 and 2014 [10,11]
Click here to view |
Although clinical trials have been and continue to be very helpful in advancing practice, [13],[14],[15],[16],[17],[18],[19],[20],[21],[22],[23] they, however, offer only limited insight into what may happen in the program implementation setting where events are subject to much more varying "un-controlled" conditions. A number of authors have described PMTCT outcomes in nontrial settings. [24],[25],[26],[27],[28] The scope of these publications vary from single hospitals to country level evaluations. Some have used models applied to local data to estimate impact. [29]
We found a study that evaluated the impact of changes of national guidelines on PMTCT outcomes in a single South African clinic. [30] This is a step in the right direction. Nigeria has only recently in December 2014, launched a new set of integrated national guidelines for HIV prevention, treatment, and care. We are yet to come across any publication from Nigeria that relates infant positivity rates to changes in ARV regimen over time. It is, therefore, imperative to evaluate how PMTCT program has performed with regards to changes in ARV regimen and accompanying MTCT rates.
We conducted a desk review of PMTCT program implementation data from 2008 to 2014. The objective was to demonstrate changes in HIV transmission rates among exposed infants as the ARV regimen changed over time.
| Methods | |  |
This was a retrospective review of routinely collected PMTCT program implementation data collected using the District Health Information Software (DHIS), from 2008 to 2014.
Study setting
Data used for this review were collected from ANCs in 682 health facilities as part of PMTCT projects funded by the President's Emergency Program for AIDS Relief, through the United States Agency for International Development (USAID) Nigeria, and implemented by FHI360 across Nigeria's 36 states and the Federal Capital Territory. The period covered was from 2008 to 2011 through the Global HIV/AIDS Initiative in Nigeria (GHAIN) project; and 2011-2014 through the Strengthening Integrated Delivery of HIV/AIDS Services (SIDHAS) project.
PMTCT program implementation in all project supported health facilities were based on the national PMTCT guidelines and protocols. The technical details of these PMTCT interventions have varied over the years as the guidelines changed. [10],[11],[12] Key changes and current interventions are summarized in Box 1.
National data collection tools/registers are used to document information from HIV-positive pregnant women and their babies: General ANC, PMTCT HIV testing and counseling, PMTCT ARV, maternal follow-up, delivery, and child follow-up registers. The delivery register is used to collect information from all HIV-positive pregnant women during labor and immediately after delivery while the child follow-up register is used to document information during the follow-up of HIV-exposed infants. The national PMTCT monthly summary form (MSF) aggregates all the key PMTCT indicators. All information on the PMTCT MSF is transcribed into the DHIS on a monthly basis.
Data source and data analysis
We used routine monitoring and evaluation aggregate data from the DHIS reported through 184 of 185 facilities supported by the GHAIN project from 2008 to 2011; and 498 of 2593 facilities supported by the SIDHAS project from 2011 to 2014. The DHIS PMTCT data set were complete for all of the facilities included in this review. Indicators of interest were a percentage of pregnant women who received various ARV regimens and infant HIV positivity rates. Chi-square test was used to compare infant positivity rates across the review period. A P < 0.05 was considered statistically significant.
Ethical considerations
All data were collected at an aggregate level and do not include any identifiers, and the data used were those collected routinely as part of PMTCT service delivery in all the study focus health facilities.
| Results | |  |
The data we reviewed indicated that the total number of pregnant women counseled and tested for HIV rose steadily from 280,123 in 2008 to 600,670 in 2014; whereas maternal HIV positivity rates varied from 4.1% in 2008, 2.9% in 2011, 3.2% in 2012, then declined steadily to 1.9% in 2014 [Figure 1].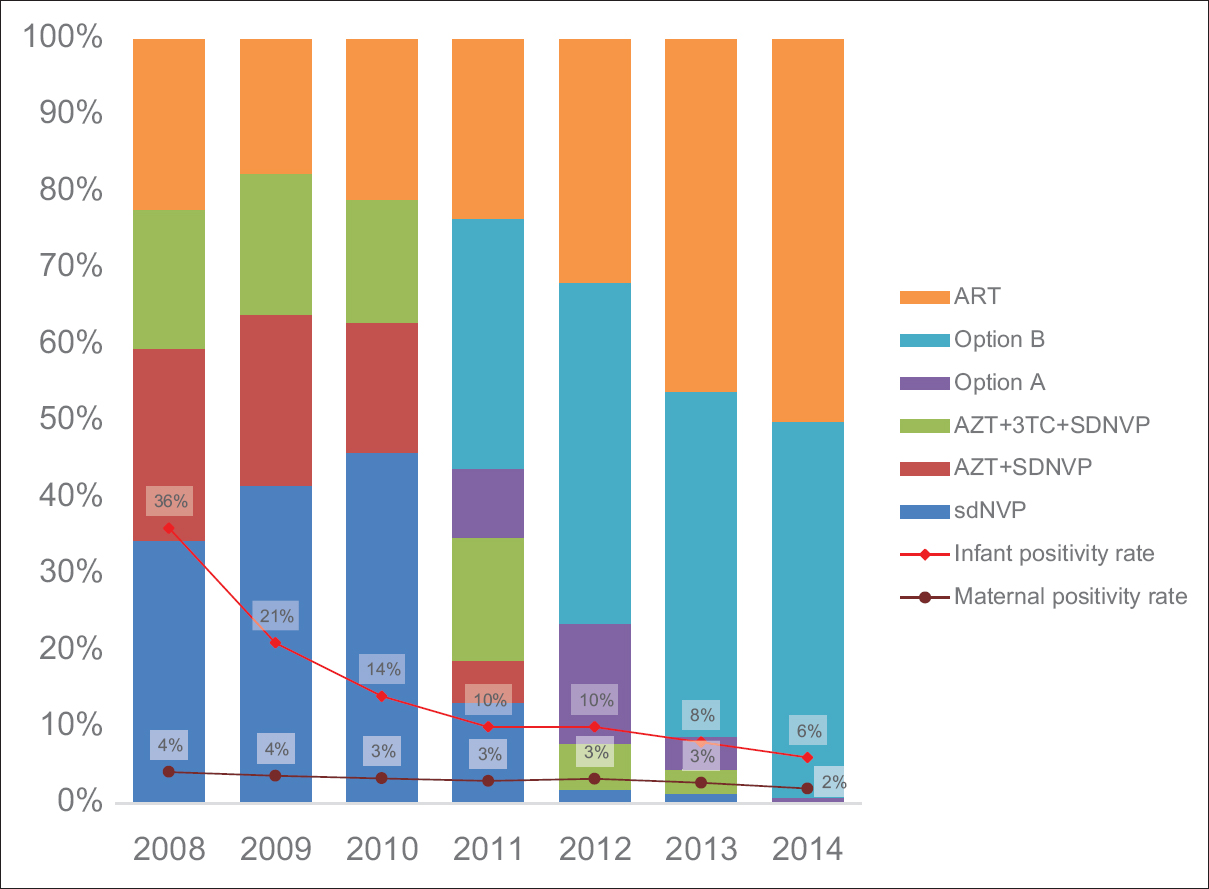 | Figure 1: Changes in infant HIV positivity rates with changes in antiretroviral regimen
Click here to view |
The total number of pregnant women who tested positive for HIV and received different ARV regimen for PMTCT during the period (2008-2014) were 63,774; ranging from 7506 in 2008 to 10,388 in 2014. Uptake of sdNVP by the positive pregnant women was 34.4%, 41.6%, and 45.9% in 2008, 2009, and 2010, respectively. In 2014 however, of all the positive pregnant women that received ARVs, 49% received triple ARV prophylaxis, whereas 50% received ART. Uptake of option A ranged from 9.0% in 2011 to 0.6% in 2014. HIV-positive pregnant women on triple ARVs (prophylaxis or treatment) increased from 22% in 2008 to 99% in 2014. Infant HIV positivity rates showed a steady decline over the years, from 38% in 2008 to 6% in 2014 (P < 0.001).
| Discussion | |  |
We observed a steady decline in the HIV positivity rates among exposed infants across our program as the country transitioned to more efficacious PMTCT ARV regimens. We also observed a steady but less steep decline in maternal HIV positivity rates within the same timeframe. The study design may not allow for direct attributions as in a controlled trial, but using data from early infant diagnosis is one of the five recommended approaches to evaluating the impact of national PMTCT programs. [31] Given the multifactorial influences that are present in everyday settings, our data show that changes in regimen coincide with a decline in positivity rates among HIV-exposed infants at the program level.
The rate of new infections among infants when considered at the population level, can be influenced by the four prongs of PMTCT described by the United Nations and included in the Nigeria national guidelines. [11],[32] These four prongs are: (1) Primary prevention of HIV infection in women of reproductive age group and their partners; (2) prevention of unintended pregnancies among HIV-positive women; (3) prevention of HIV transmission from HIV-infected mothers to their infants; and (4) care and support for HIV-infected mothers, their infants and family members. [11] It is estimated that achieving the targets for prongs 1 and 2 would contribute a 13% reduction in new infant infections. Implementing prong 3 with more effective ARV prophylaxis or treatment would contribute an additional 60% reduction, with a further 6% decrease resulting from limiting breastfeeding to 12 months. [33] When the group in focus is, however, HIV-exposed infants as considered in this paper, the positivity rates are mainly influenced by prong 3.
At a population level, the rate of decline in new infections in Nigeria is not keeping up with the rest of the world. The number of new HIV infections among children in Nigeria has declined by only 19% since 2009 compared to 50% or more in other African countries such as Botswana, Ethiopia, Ghana, Malawi, Mozambique, Namibia, South Africa, and Zimbabwe. [5] This may be due to the time lag in adopting new guidelines as well as the limited coverage of interventions. The latter possibly being a stronger factor.
Our finding of declining maternal infections in Nigeria corroborates findings in national surveys and reports from other authors. [2],[34],[35] This decline in maternal infections will ultimately contribute to a population level overall decline in new pediatric HIV infections. Successes in prongs 1 and 2 as described earlier may be contributory. [35],[36]
While changes in regimen may not be the only varying factor that has impacted the declining infant positivity rates described in this paper, we opine that it lies in the prong - prong 3 - that has witnessed the most remarkable changes during the period under consideration. The 6% positivity among the HIV-exposed babies in 2014, is a clear indication that the goal of elimination of MTCT of HIV is achievable in Nigeria. However, challenges with low ANC attendance and hospital delivery [1] must be addressed to close the gaps created by several missed opportunities within the PMTCT continuum.
A review of the 32% decline of all new infections between 2005 and 2013 reported by United Nations Programme on HIV/AIDS shows that decline in new pediatric HIV infections were 63% while adult infections have only declined by 23%. [5] This supports data presented here and presents real hope that if current efforts were sustained and coverage widened, an alignment of the country's PMTCT program with the best available scientific evidence could lead to real progress.
| Conclusions | |  |
We conclude that the evidence here discussed shows that while progress may be slow and challenges a myriad, the country is moving in the right direction with her PMTCT program. The country must therefore not rest on its oars but continue to advance its PMTCT program as well as align with available best scientific evidence.
Acknowledgment
This paper is based on data from projects implemented by FHI360 and funded by the United States President's Emergency Plan for AIDS Relief through the USAID under Cooperative Agreement No. 620-A-00-04-00-122-00 (GHAIN) and Cooperative Agreement AID-620-A-00002 (SIDHAS).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| References | |  |
| 1. | National Population Commission (NPC) [Nigeria], ICF International. Nigeria Demographic and Health Survey 2013. Abuja, Nigeria, and Rockville, Maryland, USA; 2014.  |
| 2. | Federal Ministry of Health. Technical Report on the 2010 National HIV Sero-Prevalence Sentinel Survey among Pregnant Women Attending Antenatal Clinics in Nigeria. Abuja: Federal Ministry of Health; 2010.  |
| 3. | Federal Ministry of Health. Presentation at the National PMTCT Task Team Meeting, Nasarawa, August 2013. Abuja: Federal Ministry of Health; 2013.  |
| 4. | UNAIDS. Global Plan towards the Elimination of New HIV Infections among Children by 2015 and Keeping Their Mothers Alive. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2011.  |
| 5. | UNAIDS. The Gap Report. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2014.  |
| 6. | UNAIDS. 2008 Report on the Global AIDS Epidemic. Geneva: Joint United Nations AIDS Program; 2008.  |
| 7. | World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: Towards Universal Access. Geneva: World Health Organization; 2010.  |
| 8. | Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr 2002;29:484-94.  |
| 9. | Kesho Bora Study Group, de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): A randomised controlled trial. Lancet Infect Dis 2011;11:171-80.  |
| 10. | Federal Ministry of Health. National Guidelines on Prevention of Mother-to-Child Transmission of HIV (PMTCT). Abuja: Federal Ministry of Health; 2005.  |
| 11. | Federal Ministry of Health. National Guidelines for Prevention of Mother-to-Child Transmission of HIV (PMTCT). Abuja: Federal Ministry of Health; 2010.  |
| 12. | Federal Ministry of Health. Integrated National Guidelines for HIV Prevention, Treatment and Care. Abuja: Federal Ministry of Health; 2014.  |
| 13. | Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O′Sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994;331:1173-80.  |
| 14. | Dabis F, Msellati P, Meda N, Welffens-Ekra C, You B, Manigart O, et al. 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Côte d′Ivoire and Burkina Faso: A double-blind placebo-controlled multicentre trial. DITRAME Study Group. DIminution de la Transmission Mère-Enfant. Lancet 1999;353:786-92.  |
| 15. | Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999;354:795-802.  |
| 16. | Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet 2003;362:859-68.  |
| 17. | Lallemant M, Jourdain G, Le Coeur S, Kim S, Koetsawang S, Comeau AM, et al. A trial of shortened zidovudine regimens to prevent mother-to-child transmission of human immunodeficiency virus type 1. Perinatal HIV Prevention Trial (Thailand) Investigators. N Engl J Med 2000;343:982-91.  |
| 18. | Lallemant M, Jourdain G, Le Coeur S, Mary JY, Ngo-Giang-Huong N, Koetsawang S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med 2004;351:217-28.  |
| 19. | Leroy V, Karon JM, Alioum A, Ekpini ER, Meda N, Greenberg AE, et al. Twenty-four month efficacy of a maternal short-course zidovudine regimen to prevent mother-to-child transmission of HIV-1 in West Africa. AIDS 2002;16:631-41.  |
| 20. | Mandelbrot L, Landreau-Mascaro A, Rekacewicz C, Berrebi A, Bénifla JL, Burgard M, et al. Lamivudine-zidovudine combination for prevention of maternal-infant transmission of HIV-1. JAMA 2001;285:2083-93.  |
| 21. | Petra Study Group. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): A randomised, double-blind, placebo-controlled trial. Lancet 2002;359:1178-86.  |
| 22. | Wiktor SZ, Ekpini E, Karon JM, Nkengasong J, Maurice C, Severin ST, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Côte d′Ivoire: A randomised trial. Lancet 1999;353:781-5.  |
| 23. | Leroy V, Karon JM, Alioum A, Ekpini ER, van de Perre P, Greenberg AE, et al. Postnatal transmission of HIV-1 after a maternal short-course zidovudine peripartum regimen in West Africa. AIDS 2003;17:1493-501.  |
| 24. | Coetzee D, Hilderbrand K, Boulle A, Draper B, Abdullah F, Goemaere E. Effectiveness of the first district-wide programme for the prevention of mother-to-child transmission of HIV in South Africa. Bull World Health Organ 2005;83:489-94.  |
| 25. | Lussiana C, Clemente SV, Ghelardi A, Lonardi M, Pulido Tarquino IA, Floridia M. Effectiveness of a prevention of mother-to-child HIV transmission programme in an urban hospital in Angola. PLoS One 2012;7:e36381.  |
| 26. | Plipat T, Naiwatanakul T, Rattanasuporn N, Sangwanloy O, Amornwichet P, Teeraratkul A, et al. Reduction in mother-to-child transmission of HIV in Thailand, 2001-2003: Results from population-based surveillance in six provinces. AIDS 2007;21:145-51.  |
| 27. | Townsend CL, Byrne L, Cortina-Borja M, Thorne C, de Ruiter A, Lyall H, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS 2014;28:1049-57.  |
| 28. | Anoje C, Aiyenigba B, Suzuki C, Badru T, Akpoigbe K, Odo M, et al. Reducing mother-to-child transmission of HIV: Findings from an early infant diagnosis program in south-south region of Nigeria. BMC Public Health 2012;12:184.  |
| 29. | Dube S, Boily MC, Mugurungi O, Mahomva A, Chikhata F, Gregson S. Estimating vertically acquired HIV infections and the impact of the prevention of mother-to-child transmission program in Zimbabwe: Insights from decision analysis models. J Acquir Immune Defic Syndr 2008;48:72-81.  |
| 30. | Van Schalkwyk M, Andersson MI, Zeier MD, La Grange M, Taljaard JJ, Theron GB. The impact of revised PMTCT guidelines: A view from a public sector ARV clinic in Cape Town, South Africa. J Acquir Immune Defic Syndr 2013;63:234-8.  |
| 31. | World Health Organization. A Short Guide on Methods: Measuring the Impact of National PMTCT Programmes: Towards the Elimination of New HIV Infections among Children by 2015 and Keeping their Mothers Alive. Geneva: World Health Organization; 2012.  |
| 32. | World Health Organization. Prevention of Mother-to-Child Transmission (PMTCT). Briefing Note. Geneva: World Health Organization; 2007.  |
| 33. | The Inter-agency Task Team for Prevention and Treatment of HIV Infection in Pregnant Women, Mothers, and their Children (IATT). Preventing HIV and unintended pregnancies: Strategic Framework 2011-2015. 2 nd ed. New York: IATT; 2012;2012.  |
| 34. | Imade GE, Sagay AS, Musa J, Ocheke AN, Adeniyi DS, Idighri M, et al. Declining rate of infection with maternal human immunodeficiency virus at delivery units in north-central Nigeria. Afr J Reprod Health 2013;17:138-45.  |
| 35. | UNAIDS. 2013 Report on the Global AIDS Epidemic. Geneva: Joint United Nations AIDS Program; 2013.  |
| 36. | Ahmed S, Li Q, Liu L, Tsui AO. Maternal deaths averted by contraceptive use: An analysis of 172 countries. Lancet 2012;380:111-25.  |
[Figure 1]
[Table 1]
|