|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2015 | Volume
: 3
| Issue : 2 | Page : 41-46 |
|
Assessment of Metabolic syndrome among adult human immunodeficiency virus/acquired immunodeficiency syndrome patients in a tertiary health facility in Southeast Nigeria
Victoria N Uwanuruochi1, Favour S Michael2, Kelechukwu Uwanuruochi1, Christian Okafor3, Esther N Ofoegbu3, Basden J Onwubere3, John M Oli3
1 Department of Medicine, Federal Medical Centre, Umuahia, Nigeria
2 Department of Medical Laboratory, Federal Medical Centre, Umuahia, Nigeria
3 Department of Medicine, University of Nigeria Teaching Hospital, Enugu, Nigeria
| Date of Web Publication | 14-Jul-2016 |
Correspondence Address:
Kelechukwu Uwanuruochi
Department of Medicine, Federal Medical Centre, Umuahia, PMB 7001
Nigeria
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/2321-9157.186352

Context: Metabolic syndrome (MS) on the background of human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome has not been reported from Southeast Nigeria. Aims: We sought to determine the prevalence of MS among HIV-infected Nigerians seen at the Federal Medical Centre, Umuahia, its correlation with highly active antiretroviral therapy (HAART) and other demographics. A total of 105 HAART-treated and 60 HAART-naοve patients were reviewed. Settings and Design: This study was cross-sectional, and the data were prospectively collected. Methodology: They were matched for sex and age. Anthropometric data including current weight and height, waist circumference, blood pressure, as well as blood lipids and fasting glucose were measured. MS was determined using National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III criteria. Statistical Analysis Used: SPSS version 17.0 (Chicago IL, USA) was used for data collection and analysis. Results: There was 24.3% overall prevalence of MS in the study population, 28.8% in HAART-treated, and 25% in HAART-naïve patients (P = 0.554). The prevalence of MS components was as follows: hypertension (49.7%), impaired fasting glucose (9.62%), hypertriglyceridemia (32.0%), low high-density lipoprotein-cholesterol (44.2%), and central obesity (22.1%). Correlation of MS with female gender was highly significant (r = −0.306, P = 0.002). Conclusions: MS was not significantly associated with the use of HAART in our patients but correlated with female gender. Keywords: Correlation with highly active antiretroviral therapy, human immunodeficiency virus/acquired immunodeficiency syndrome, metabolic syndrome, Southeast Nigeria, tertiary hospital
How to cite this article:
Uwanuruochi VN, Michael FS, Uwanuruochi K, Okafor C, Ofoegbu EN, Onwubere BJ, Oli JM. Assessment of Metabolic syndrome among adult human immunodeficiency virus/acquired immunodeficiency syndrome patients in a tertiary health facility in Southeast Nigeria. J HIV Hum Reprod 2015;3:41-6 |
How to cite this URL:
Uwanuruochi VN, Michael FS, Uwanuruochi K, Okafor C, Ofoegbu EN, Onwubere BJ, Oli JM. Assessment of Metabolic syndrome among adult human immunodeficiency virus/acquired immunodeficiency syndrome patients in a tertiary health facility in Southeast Nigeria. J HIV Hum Reprod [serial online] 2015 [cited 2018 Aug 6];3:41-6. Available from: http://www.j-hhr.org/text.asp?2015/3/2/41/186352 |
| Introduction | |  |
Metabolic syndrome (MS) has been reported among patients with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS), and more so among patients with HIV infection who are on highly active antiretroviral therapy (HAART). Most studies have been carried out in the developed nations, [1],[2],[3],[4],[5] with few local studies. [6],[7] This would be the first report from the Southeast Nigeria.
Jayasuriya et al. [8] studied MS among black African patients in the United Kingdom. The prevalence of MS was 22.1% by International Diabetes Federation (IDF) criteria and 15.5% by National Cholesterol Education Program (NCEP) criteria. Muhammad et al. [7] described the cardiovascular risk profile of HIV/AIDS patients receiving HAART at a health facility in Northern part of Nigeria. Twenty-one percent of their patients on HAART had MS compared with 9% of HAART-naïve patients. They had defined MS using the NCEP adult treatment panel III (ATP III) guidelines. From Ogbomoso, Southwest Nigeria, Ayodele et al. [6] looked at the prevalence and clinical correlates of MS among an HIV-infected outpatient population using the NCEP-ATP III, the IDF, and the joint interim statement (JIS) definitions. The prevalence rates of MS according to the ATP III, IDF, and JIS criteria were 12.7%, 17.2%, and 21.0%, respectively. In their study, MS was significantly associated with female gender (all definitions), body mass index (BMI) (all definitions), increasing age, and CD4 count (IDF definition). There was no significant association between MS and HAART. They conclude that the prevalence of MS in HIV/AIDS varied with the criteria used and that MS correlates with traditional cardiovascular risk factors rather than HAART-related factors.
| Methodology | |  |
We set out to determine the prevalence of MS in our HAART-treated and HAART-naïve patients, and to determine any association of MS with the different drug combinations, duration of treatment, and other clinical variables such as age, gender, occupation, alcohol consumption, level of education, social class, and physical activity. Our study design was cross-sectional.
The location of the study was the ART clinic, of the Federal Medical Centre (FMC), Umuahia, Nigeria.
The sample size was determined using Fisher's formular [9] n = z2pq/d2 , where n = minimum sample size, z = 95% confidence level, i.e., 1.96, d = level of precision (0.05), P = the proportion in the target population estimated to have a particular characteristic, i.e., maximum prevalence reported in a study of a similar population (22.1%), and q = the proportion in the target population estimated to lack a particular characteristic, i.e., (1.0 − p). [9] The prevalence of MS in black Africans with HIV/AIDS was reported as 22.1%. [8] Therefore, the calculated minimum sample size was 118. Patients were recruited from the ART clinic of FMC, Umuahia, during the period of the study. All eligible patients were recruited if they gave consent. The study was reviewed and approved by the FMC, Umuahia, Health Research Ethics Committee.
The inclusion criteria were (1) patients of both sexes aged 18 years and above as at their last birthday, with confirmed HIV positive serology, were eligible. The lower limit of 18 years was used to reflect the age limit for classification of an adult in Nigeria in accordance with the child rights Act adopted by the Federal Government in May 2003. [10] (2) Regular attendance to the ART clinic, of FMC, Umuahia, i.e., using their appointment cards as a check. (3) For the subgroup on HAART, regular treatment for at least 1 year. (4) Obtained informed consent. Patients below 18 years of age as at the time of the study using their last birthday or estimated birthday, patients on chronic corticosteroids use, pregnant females based on their last menstrual period, patients who withdrew from combination HAART, patients with any active opportunistic infection or neoplasia and those with type 1 diabetes mellitus (DM), liver, or heart failure, patients with a history of abuse of tobacco, and other psychoactive agent, for example, cocaine, and patients who were unwilling to give their consent were all excluded from the study. About 140 patients of similar age and sex distributions were recruited from the ART clinic. One-hundred and five patients were HAART-treated, whereas 60 were HAART-naive.
The clinical evaluation and collection of samples were carried out at the ART clinic of the FMC, Umuahia, between October 2011 and April 2012. The patients were invited to participate in this cross-sectional study. Those who responded were interviewed with a questionnaire by the investigator using a standard pro forma to obtain demographic data and clinical history. The evaluation included asking for a history of hypertension, DM, tobacco and alcohol use, and medication history including use and duration of ART. Three categories of social class (high, middle, and low) were defined based on the public service classification of occupations (respective civil servants categories: Upper, middle, and lower). [11]
Patients working for private firms or for themselves were allocated to these categories based on their profession. Homemakers were attributed the occupation category of their husbands. [11] Alcohol consumption data were collected during the clinic interviews by asking whether the person "ever consumed alcoholic beverages" or "presently drinks alcoholic beverages at all;" if the latter, subjects were asked specifically about the number of drinks per week of each type of beverage (beer, wine, and spirits). [12] For purposes of the study, a "drink" was defined as a bottle of beer (12.6 g of alcohol), a glass of wine (13.2 g of alcohol), or a shot of spirits (15 g of alcohol). Mild, moderate, or high intake was defined as <5, 5-30, and >30 g/day, respectively. [11] Patients were classified as nonsmoker (has never smoked), previous smokers, and current smokers. Subjects were considered current smokers if they smoked cigarettes at the time of the interview, previous smokers if they were not current smokers but had smoked 100 cigarettes in their entire life, and nonsmokers if they smoked less than this amount. [13] Physical activity was graded as none (<4 times/month), low (4-10 times/month), moderate (11-19 times/month), or high (>19 times/month) based on the monthly frequency of engaging in leisure time physical activities such as walking for ≥1.6 km, jogging, swimming, cycling, aerobics, sports, gardening, and weight training. [13]
The height and weight were measured, and the BMI (kg/m 2 ) was calculated as the ratio of measured weight to the square of the measured height. Waist circumference (WC), hip circumference, and blood pressure were measured according to standard recommendations. [11] HIV screening was by enzyme immunoassay and confirmatory test by Western-Blot electrophoresis. The CD4 cell counts were obtained using the flow cytometry method. Venous samples were collected to estimate plasma glucose, triglyceride (TG), total cholesterol (TC), and high-density lipoprotein-cholesterol (HDL-C) fraction after an overnight fast of 6-12 h. TG, TC, and HDL-C levels were measured enzymatically. HDL-C was determined after the precipitation of the low-density lipoprotein (LDL) fraction, whereas LDL-cholesterol LDL-C was calculated using Friedewald formula [14] LDL = (TC − HDL-C) − TG/2. Fasting plasma glucose (FPG) was estimated using the glucose oxidase method. The samples were analyzed at the central laboratory of the hospital by the same medical laboratory scientist.
MS was defined according to the NCEP-ATP III definition [15] based on three or more of the following: TG ≥1.7 mmol/l (150 mg/dL), FPG ≥6.1 mmol/l (110 mg/dL), WC > 94 cm in men or > 80 cm in women, systolic blood pressure ≥ 130 or diastolic blood pressure ≥85 mmHg or treatment for hypertension and HDL-C <40 mg/dL (1.03 mmol/L) in males <50 mg/dL (1.29 mmol/L) in females, or specific treatment for this lipid abnormality. For data collection and analysis, SPSS version 17.0 (SPSS Inc, Chicago Illinois, USA) was used. Descriptive statistics were computed with standard methods and are presented as mean and standard deviations. Chi-square test was used to compare the association between categorical variables, and independent t-test was used to compare the mean value of some laboratory parameters and sociodemographic variables. A P < 0.05 was considered to be statistically significant.
| Results | |  |
A total of 165 adults were enrolled into the study. They comprised 105 patients HAART-treated and 60 HAART-naïve patients. The patients included artisans (21.8%), traders (36.4%), students (1.9%), civil servants (21.8%), unemployed (2.4%), farmers (11.7%), and others which included missing data (3.9%). Demographic, clinical, and biochemical characteristics are as shown in [Table 1]. The prevalence of MS and its various components are as recorded in [Table 2]. Of the variables correlated with MS, (CD4 count, gender, age, occupation, level of education, social class, duration of illness, consumption alcohol, smoking, physical activity, and class of antiretroviral) only the correlation of MS with CD4 count (r = −0.265, P = 0.024) and gender (r = −0.306, P = 0.002) were statistically significant. When WC was controlled, the correlation between MS and CD4 count was no longer significant (r = 0.217, P = 0.071). The variables of age, CD4 counts, and gender were also compared between those with and without MS [Table 3].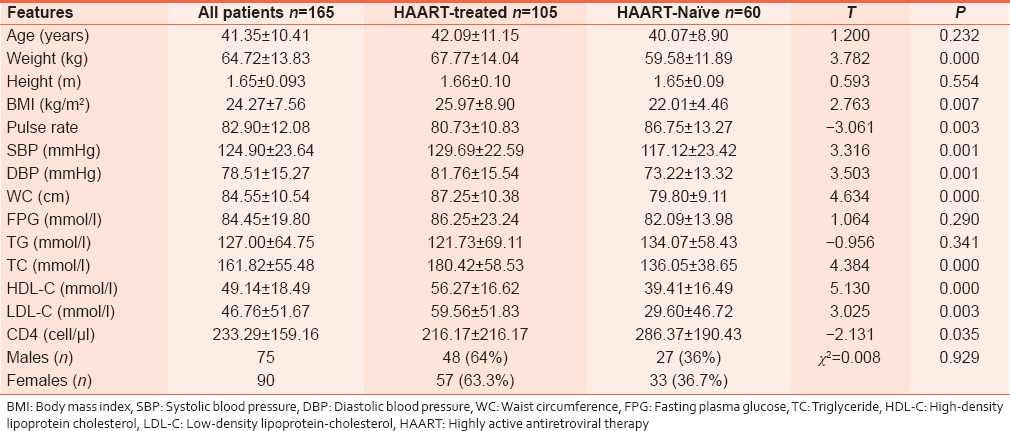 | Table 1: Demographic, clinical, and biochemical features of the study population
Click here to view |
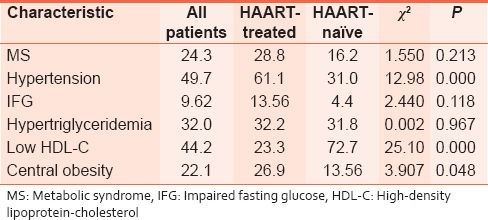 | Table 2: Prevalence (percentage) of Metabolic syndrome and its various components
Click here to view |
The distribution of the HAART-treated patients to various combinations was as follows: Combivir-efavirenz 1 (0.6%), Combivir-nevirapine 97 (58.8%), truvada-nevirapine 5 (3.0%), truvada-efavirenz 1 (0.6%), and kaletra -truvada 1 (0.6%). Combivir contained lamivudine and zidovudine, truvada contained emtricitabine and tenofovir, and kaletra contained lopinavir and ritonavir. The vast majority of the patients were on one of the antiretroviral drug combinations, so no significant associations could be pursued.
| Discussion | |  |
The prevalence of MS was 24.3% in the population studied. A study on apparently healthy adults from our center had reported the MS prevalence of 24.7%. [16] This suggests HIV-infection in our center is not associated with a higher prevalence of MS. From Northeastern Nigeria, Muhammad et al. [7] reported a prevalence of 15%, whereas Ayodele et al. [6] in Southwestern Nigeria found 12.7%, both using ATP III guidelines. The mean age in our patients 41.4 ± 10.41 was higher than that of in the patients studied by Sanusi et al. (32.5 ± 7.55), which would contribute significantly to the lower prevalence reported by them. As in other locally reported studies, [6],[7] MS was strongly correlated with female gender. When the sexes were compared, central obesity and low HDL were significantly more prevalent in females. Even in apparently healthy Nigerians, [17] the prevalence of MS and obesity has been reported to be higher in female (20.9%; 31.0%) than male traders (6.5%; 7.1%), respectively. Elsewhere, higher rates of obesity have been reported among black women. [18]
Environmental and sociocultural factors that contribute to increased obesity in Nigerian women will include engagement in occupations involving less physical activity, [19] insecurity of life, and heavy traffic which discourage physical exercise and walking. [20]
Sani et al. in their study of apparently healthy Nigerians also reported a higher prevalence of low HDL-C in females, [21] with the prevalence to be 51.9% in males but 65% in females (P < 0.05). It will also be observed that central obesity and low HDL-C, the two conditions significantly elevated in females, were the ones defined in ATP-III by gender-specific criteria. The optimal cut-off value of WC has been noted to vary across different ethnicities, but as yet accepted criteria have not been locally developed. [22],[23] The same controversy trails are low-HDL. Ogbu and Ugwuja [24] have suggested modifications in the ATP III criteria for the diagnosis of the MS in Nigerians including the use of ≤1.3 mmol as cutoff for HDL-C and ≥93 cm as cutoff for WC in both men and women.
MS also correlated with CD4 count level in our study. The CD4 level was lower in those without MS. However, this difference was related to loss in the body weight as a result of chronic HIV infection (resulting in fewer numbers meeting the criteria). The correlation became insignificant when we controlled for WC. MS was not correlated with ART. This is expected since only 0.6% of the patients on HAART were on protease inhibitors.
Lipodystrophy must also be mentioned as another of the metabolic changes in HIV individuals, apart from dyslipidemia and insulin resistance. [25] HAART regimens, especially those including protease inhibitors, have been implicated in the pathogenesis. [26] The clinical lipodystrophy comprises both fat accumulation and fat wasting, with the fat wasting consisting of subcutaneous fat atrophy in the legs, buttocks, arms, and face. It is important because lipodystrophy on its own portends metabolic implications equivalent to those of MS. [27] This will however be noted as a limitation in our methodology, which did not report peripheral fat distribution. This study had other limitations. The NCEP-ATP III criteria, which were derived in North America, may not correctly define MS in Africans. Only 0.6% of the patients studied were on protease inhibitors. The impact of wasting syndrome in HAART-naοve patients, which has been reported more in Sub-Saharan Africa, was not analyzed. [28]
| Conclusions | |  |
We report on the MS in HIV/AIDS patients seen in Umuahia, Southeast Nigeria. There was 24.3% overall prevalence of MS, with 28.8% in HAART-treated and 25% in HAART-naïve patients (P = 0.554). The prevalence of MS components was as follows: Hypertension (49.7%), impaired fasting glucose (9.62%), hypertriglyceridemia (32.0%), low HDL-C (44.2%), and central obesity (22.1%). Correlation of MS with female gender was highly significant (r = −0.306, P = 0.002).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| References | |  |
| 1. | Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: Associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care 2007;30:113-9.  |
| 2. | Noor MA, Lo JC, Mulligan K, Schwarz JM, Halvorsen RA, Schambelan M, et al. Metabolic effects of indinavir in healthy HIV-seronegative men. AIDS 2001;15:F11-8.  |
| 3. | Gazzaruso C, Sacchi P, Garzaniti A, Fratino P, Bruno R, Filice G. Prevalence of metabolic syndrome among HIV patients. Diabetes Care 2002;25:1253-4.  |
| 4. | Bonfanti P, Giannattasio C, Ricci E, Facchetti R, Rosella E, Franzetti M, et al. HIV and metabolic syndrome: A comparison with the general population. J Acquir Immune Defic Syndr 2007;45:426-31.  |
| 5. | Bruno R, Gazzaruso C, Sacchi P, Zocchetti C, Giordanetti S, Garzaniti A, et al. High prevalence of metabolic syndrome among HIV-infected patients: Link with the cardiovascular risk. J Acquir Immune Defic Syndr 2002;31:363-5.  |
| 6. | Ayodele OE, Akinboro AO, Akinyemi SO, Adepeju AA, Akinremi OA, Alao CA, et al. Prevalence and clinical correlates of metabolic syndrome in Nigerians living with human immunodeficiency virus/acquired immunodeficiency syndrome. Metab Syndr Relat Disord 2012;10:373-9.  |
| 7. | Muhammad S, Sani MU, Okeahialam BN. Cardiovascular disease risk factors among HIV-infected Nigerians receiving highly active antiretroviral therapy. Niger Med J 2013;54:185-90.  [ PUBMED]  |
| 8. | Jayasuriya A, Allan P, Wade A, Das S. Metabolic syndrome in black African HIV patients. Which criteria should we use? Int Cong Drug Ther HIV 2006;125:12-6.  |
| 9. | Araoye MO. Research Methodology with Statistics for Health and Social Sciences. Ilorin: Nathadex Publishers; 2003:117-21.  |
| 10. | Alemika EE, Chukwuma I, Lafratta D, Messerli D, Souckova J. Rights of the Child in Nigeria. Report on the Implementation of the Convention on the Rights of the Child by Nigeria. World Organisation against Torture (Organisation Mondiale Contre la Torture), Geneva, Switzerland. Available from: http://www.who.int/chp/steps. [Last retrieved on 2014 Apr 07].  |
| 11. | Fezeu L, Minkoulou E, Balkau B, Kengne AP, Awah P, Unwin N, et al. Association between socioeconomic status and adiposity in urban Cameroon. Int J Epidemiol 2006;35:105-11.  |
| 12. | Djoussé L, Arnett DK, Eckfeldt JH, Province MA, Singer MR, Ellison RC. Alcohol consumption and metabolic syndrome: Does the type of beverage matter? Obes Res 2004;12:1375-85.  |
| 13. | St-Onge MP, Janssen I, Heymsfield SB. Metabolic syndrome in normal-weight Americans: New definition of the metabolically obese, normal-weight individual. Diabetes Care 2004;27:2222-8.  |
| 14. | Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502.  |
| 15. | Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97.  |
| 16. | Uwanuruochi K, Ukpabi OJ, Onwuta CN, Onwubere BJ, Anisiuba BC, Michael FS. Cardiovascular risk factors in adult staff of Federal Medical Centre, Umuahia: A comparison with other Nigerian studies. West Afr J Med 2013;32:243-7.  |
| 17. | Mabel AC, Arinola OA, Fasanmade AA, Olaniyi JA, Oyewole OE, Owolabi MO, et al. Indices of metabolic syndrome in 534 apparently healthy Nigerian traders. J US China Med Sci 2012;9:91-100.  |
| 18. | Ervin RB. Prevalence of Metabolic Syndrome among Adults 20 Years of Age and Over, by Sex, Age, Race and Ethnicity, and Body Mass Index: United States, 2003-2006. National Health Statistics Reports; No. 13. Hyattsville, MD: National Centre for Health Statistics; 2009.  |
| 19. | Afolabi WA, Addo AA, Sonibare MA. Activity pattern, energy intake and obesity among Nigerian urban market women. Int J Food Sci Nutr 2004;55:85-90.  |
| 20. | Adewale LO, Babatunde OA, Adetoyeje YO, Benedicte D, Ilse D, James FS. Environmental factors associated with overweight among adults in Nigeria. Int J Behav Nutr Phys Act 2012;9:32.  |
| 21. | Sani MU, Wahab KW, Yusuf BO, Gbadamosi M, Johnson OV, Gbadamosi A. Modifiable cardiovascular risk factors among apparently healthy adult Nigerian population - A cross sectional study. BMC Res Notes 2010;3:11.  |
| 22. | Misra A, Wasir JS, Vikram NK. Waist circumference criteria for the diagnosis of abdominal obesity are not applicable uniformly to all populations and ethnic groups. Nutrition 2005;21:969-76.  |
| 23. | Wang Z, Ma J, Si D. Optimal cut-off values and population means of waist circumference in different populations. Nutr Res Rev 2010;23:191-9.  |
| 24. | Ogbu IS, Ugwuja EI. Metabolic syndrome in hypertensive Nigerians: Risk factor analysis. IOSR J Pharm Biol Sci 2012;4:28-32.  |
| 25. | Biron A, Bobin-Dubigeon C, Volteau C, Piroth L, Perré P, Leport C, et al. Metabolic syndrome in French HIV-infected patients: Prevalence and predictive factors after 3 years of antiretroviral therapy. AIDS Res Hum Retroviruses 2012;28:1672-8.  |
| 26. | Carr A, Workman C, Carey D, Rogers G, Martin A, Baker D, et al. No effect of rosiglitazone for treatment of HIV-1 lipoatrophy: Randomised, double-blind, placebo-controlled trial. Lancet 2004;363:429-38.  |
| 27. | Barbaro G, Iacobellis G. Metabolic syndrome associated with HIV and highly active antiretroviral therapy. Curr Diab Rep 2009;9:37-42.  |
| 28. | Koethe JR, Heimburger DC. Nutritional aspects of HIV-associated wasting in Sub-Saharan Africa. Am J Clin Nutr 2010;91:1138S-42S.  |
[Table 1], [Table 2], [Table 3]
|