|
 
 |
| TECHNICAL REPORT |
|
| Year : 2015 | Volume
: 3
| Issue : 2 | Page : 47-55 |
|
Integrated national guidelines for HIV prevention, treatment, and care: Chapters 5 and 6
Federal Ministry of Health (Nigeria)
Federal Republic of Nigeria, Federal Ministry of Health, Abuja, Nigeria
| Date of Web Publication | 14-Jul-2016 |
Correspondence Address:
Federal Ministry of Health (Nigeria)
Federal Republic of Nigeria, Federal Ministry of Health, Abuja
Nigeria
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/2321-9157.186353

These guidelines were developed taking into consideration the guiding principles of the WHO 2013 consolidated guidelines on the use of antiretrovirals (ARVs) and the President's Comprehensive Response Plan for HIV/AIDS in Nigeria. It is intended to fast-track the achievement of universal access to HIV prevention, treatment, care, and support in Nigeria. Guiding principles of these Guidelines includes the followings: (a) Public health approach - In line with the National scale-up strategy of decentralization and integration, these guidelines are based on a public health approach to scaling up the use of ARV drugs for HIV treatment and prevention. The public health approach will ensure access to high-quality services at all levels of the health-care system including the community and primary health care settings, with a focus on the best practices that are commensurate with available resources at all levels. (b) Implementation based on national context - Implementation of the recommendations in these guidelines will be informed by national context, including HIV epidemiology, availability of resources, the organization and capacity of the health system, and anticipated cost-effectiveness. While aiming to achieve attainment of the global milestones, the best practices within the country will be further refined, promoted, implemented, and scaled up nationwide. (c) Strengthening health systems through innovation and learning - Strengthening health systems recommended and described in these guidelines will be implemented with a view to strengthening the continuum of HIV care and broader health systems, especially primary care and chronic care. As more lessons are learned from ongoing integration and decentralization of HIV services at lower-level health facilities, implementation is encouraged and findings widely disseminated. Keywords: Antiretroviral therapy, Guideline, HIV, Nigeria, prevention of mother-to-child transmission
How to cite this article:
Federal Ministry of Health (Nigeria). Integrated national guidelines for HIV prevention, treatment, and care: Chapters 5 and 6. J HIV Hum Reprod 2015;3:47-55 |
How to cite this URL:
Federal Ministry of Health (Nigeria). Integrated national guidelines for HIV prevention, treatment, and care: Chapters 5 and 6. J HIV Hum Reprod [serial online] 2015 [cited 2018 Aug 6];3:47-55. Available from: http://www.j-hhr.org/text.asp?2015/3/2/47/186353 |
| Chapter 5: Prevention Of Mother-To-Child Transmission Of HIV Infection | |  |
Mother-to-child transmission of HIV
Most children <15 years living with HIV acquire the infection through mother-to-child transmission (MTCT). This can occur during pregnancy, labor, and delivery or breastfeeding. In the absence of interventions, the risk of MTCT is 25-40%.
The high burden of MTCT of HIV in Sub-Saharan Africa including Nigeria is due to high rates of heterosexual transmission, high prevalence of HIV in women of reproductive age, high total fertility rate, characteristically prolonged breastfeeding culture, suboptimal infection prevention measures during labor and delivery, and limited access to general HIV prevention interventions. Transmission of HIV in children has become a critical health challenge that threatens to undermine the gains of child survival strategies in the African continent.
Risk factors for mother-to-child transmission
The rate of MTCT of HIV is affected by many factors. These have been grouped into viral, maternal, obstetric, fetal, and breastfeeding factors [Table 1].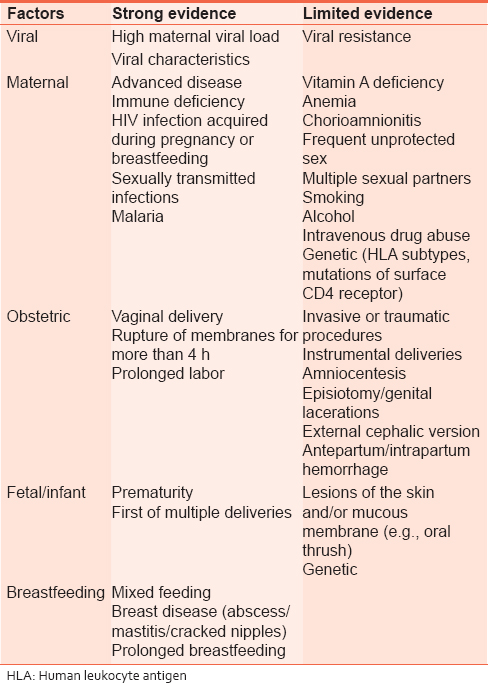 | Table 1: Factors associated with increased risk of mother - to - child transmission
Click here to view |
Comprehensive approach to primary prevention of mother-to-child transmission
The prevention of MTCT (PMTCT) of HIV involves all persons of reproductive age group. It is based on the globally accepted four pronged approaches:
- Primary prevention of HIV infection in women of reproductive age and their partners
- Prevention of unintended pregnancies among HIV-positive women
- Prevention of HIV transmission from infected mothers to their infants
- Provision of appropriate treatment, care, and support to HIV-infected mothers, their infants, and family.
Primary prevention of HIV infection in women of reproductive age and their partners using a combination approach which includes the following:
- Targeted information and education including the use of the "ABC" approach to enhance safer and responsible sexual behavior and practices which covers:
- Delaying the onset of sexual activity until marriage
- Practicing abstinence
- Reducing the number of sexual partners
- Consistent and correct use of condoms
- Provision of early diagnosis and treatment of sexually transmitted infections (STIs): The early diagnosis and treatment of STIs can reduce the incidence of HIV in the general population by about 40%. Comprehensive STI treatment services present an opportunity to provide information on HIV infection, MTCT, and referral for testing and counseling
- Making HIV testing and counseling widely available: HIV testing and counseling services need to be made available to all women of childbearing age because PMTCT interventions depend on the woman knowing her HIV status
- Provision of appropriate counseling for women who are HIV negative: Counseling provides an opportunity for a woman who is HIV negative to better understand how to protect herself and her infant from HIV infection. It can also serve as powerful motivation to adopt safer sex practices, encourage partner testing, and discuss family planning (FP). School-based sex education and counseling also play an important role.
These measures should be specifically promoted for all women who test HIV negative during antenatal, labor, delivery, and postnatal care (ANC).
Prevention of unintended pregnancies among HIV-positive women
The responsibility of the government and health services is to provide HIV-positive women and their partners with comprehensive information and education about the risks associated with childbearing as part of routine public information about HIV and AIDS. This is to ensure that HIV-positive women and their partners have informed choices of action and to respect and support the decisions they reach. This implies:
- Providing good quality, user-friendly, and easily accessible FP services so that HIV-positive women can avoid pregnancy if they choose
- Promoting condom (male/female) use combined with a more effective method of contraception (dual method) for dual protection from HIV and other STIs and from unplanned pregnancies as an effective strategy to prevent HIV transmission
- Integrating dual protection messages into FP counseling services
- Offering contraception to all HIV-positive mothers in the immediate postpartum period. To prevent unintended pregnancy, because lactational amenorrhea does not guarantee adequate contraception even in women who exclusively breastfeed.
Prevention of HIV transmission from infected mothers to their infants
- HIV testing and counseling
- HIV and infant feeding counseling
- Modification of obstetric practices
- Administration of antiretroviral therapy (ART) to eligible women
- Administration of antiretroviral (ARV) prophylaxis to mother-child pair for women not eligible for highly active ART (HAART).
Provision of appropriate treatment, care, and support to HIV-infected mothers, their infants, and family
· Package of services for mothers
- ART for women eligible for treatment
- Co-trimoxazole (CTX) prophylaxis
- Tuberculosis (TB) screening, prophylaxis, and treatment
- Continued infant feeding counseling and support
- Nutritional counseling and support
- Sexual and reproductive health services including FP
- Cervical cancer screening ( Pap smear More Details)
- Psychosocial support
- Partner counseling and testing
· Package of services for HIV-exposed children:
- ARV prophylaxis
- Routine immunization and growth monitoring and support
- CTX prophylaxis starting at 6 weeks
- HIV diagnostic testing (early infant diagnosis) at 6-8 weeks of age and 6 weeks after breastfeeding has ended. Virologic test is to be used where available; HIV antibody test can be used for HIV diagnosis for children older than 9 months where virologic test is not available. HIV antibody test is the recommended diagnostic testing strategy for children older than 18 months
- HIV antibody tests should primarily be used for screening of infants and children <18 months so as to establish exposure status where the mother has not herself been tested for HIV or is not willing to be tested
- Ongoing infant feeding counseling and support
- Screening and management of TB
- Prevention and treatment of malaria
- Nutritional care and support
- Psychosocial care and support
- ARV therapy for eligible HIV-infected children [Chapter 4]
- Symptom management and palliative care if needed
- The success of the interventions depends on strategies that will address stigma and discrimination as well as those that will strengthen referral, adherence, retention, and community mobilization.
Antenatal care for HIV-positive women
When a woman is known to be HIV positive or is diagnosed as HIV positive during pregnancy, her obstetric and medical care will need to be strengthened and modified. Posttest counseling for HIV-positive pregnant women should include information on the following:
- Disclosure, partner notification, and testing
- Benefits of PMTCT intervention
- ART
- Nutrition
- Delivery
- Infant feeding and infant testing
- Importance of testing other children and benefits of pediatric ART
- Need for follow-up and adherence.
All HIV-positive women should be given optimal health care to ensure their safe delivery. In a situation where the life of the woman is being threatened by the continuation of the pregnancy, termination of pregnancy should be in accordance with the provisions of the law.
All pregnant, HIV-positive women must be screened for TB at ANC. TB screening should include the following:
- Ask the patients about cough, weight loss, fever, and night sweats
- Check for lymph node enlargement.
Initial examination
An HIV-infected pregnant woman should have a full physical examination. This should focus on HIV-related illnesses including symptoms and signs of Opportunistic infections (Ols) (especially TB). Special attention should be paid to the following:
- Anemia
- Persistent diarrhea
- Respiratory infections: TB is a common one and other bacterial respiratory infections are common in HIV-positive women
- Oral and vaginal candidiasis
- Lymphadenopathy
- Herpes zoster (chronic/recurrent) is a common presenting sign of HIV infection, occurring early in the disease, often before there is much immune suppression
- Other skin conditions such as candidiasis and vaginal wart
- Other STIs
- Weight gain or loss.
Laboratory investigations
HIV-positive pregnant women should be tested for syphilis (venereal disease research laboratory) and have hemoglobin or hematocrit estimation and urinalysis done. CD4 + cell count should be ordered before commencement of ARVs for baseline assessment and ARVs initiated once the sample has been taken. CD4 + cell count should also be repeated before exit from PMTCT services. [Table 2] summarizes the recommended laboratory investigations: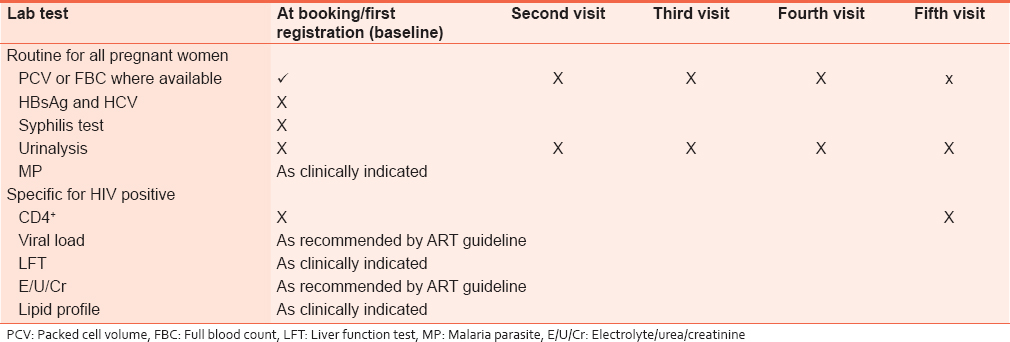 | Table 2: Recommended laboratory investigations for HIV - positive pregnant women
Click here to view |
Management of HIV-positive women in labor
Labor management should follow usual obstetric guidelines. Analgesia should be given in labor if required and epidural analgesia is not contraindicated [Table 3].
HIV-positive women should not be isolated or treated differently from other women in labor. Supportive measures, empathy, and caring attitudes by the health-care provider are important for all women, particularly for the HIV-infected woman concerned about her condition and risks of HIV transmission to the child. There should be no room for stigmatization and discrimination by medical staff including for those who may not have disclosed their status to their partner or family members.
Whenever possible, during labor, HIV-positive women should have the option to have a companion of their choice who knows their HIV status and can provide supportive companionship. Where this is not possible, labor ward staff must be sensitive to the fears and concerns of the HIV-positive mother about her infection and how much she had told any of her companions.
Induction of labor
As prolonged rupture of membranes is associated with increased risk of MTCT, careful assessment of the desirability of cesarean section (CS) rather than induction of labor is necessary. Where induction is chosen, membranes should be left intact for as long as possible. Oxytocin should not be used with intact membranes. The use of prostaglandins or its analogs can be considered.
Conduct of delivery
Delivery should be conducted using standard practices and aseptic techniques while avoiding unnecessary trauma or prolongation of the second stage.
Vaginal delivery
HIV-positive women who are on HAART or ARV prophylaxis should be allowed to deliver vaginally where there is no obstetric contraindication. Vaginal delivery remains the primary delivery mode of choice even where it is not possible to initiate any ARV prophylaxis due to the high mortality/morbidity risks of emergency CS.
Cesarean section(s)
HIV infection on its own is not an indication for CS. Available evidence shows that elective CS for women on ART who have achieved viral suppression has no added advantage over vaginal delivery.
Elective CS can be considered for HIV-positive women before the onset of labor or rupture of membranes in cases when the woman is not on ART and/or where the maternal viral load is known to be high. Indeed, available evidence shows that when elective CS is performed before the onset of labor or rupture of membranes, it reduces the risk of MTCT by >50% as compared to vaginal delivery among women not on ART or with high viral load. [1] These guidelines, however, do not recommend routine offer of elective CS for any group of HIV-positive pregnant women.
Where CS is performed (elective or emergency) in HIV-positive women, they should receive prophylactic antibiotics. If CS is performed after prolonged labor or prolonged rupture of membranes, longer courses of antibiotics should be provided.
Specific modification of obstetric care for HIV-positive women
- Avoid invasive procedures such as chorionic villous sampling, amniocentesis or cordocentesis
- External cephalic version may carry a risk of HIV transmission to the fetus and should therefore be avoided
- Care should be individualized in special circumstances such as premature rupture of membranes (preterm and term) and antepartum hemorrhage
- Use of the partograph: Proper and consistent use of the partograph in monitoring the progress of labor will improve the management and reduce the risk of prolonged labor in all women
- Artificial rupture of membranes (ARM) is practiced routinely in many settings although it should be reserved for women with abnormal progress of labor. Rupture of membranes of >4 h duration is associated with an increased risk of HIV transmission. Therefore, early ARM should be reserved for those with fetal distress or abnormal progress. ARM can be done if cervical dilatation is 7 cm or more
- Instrumental delivery: Forceps and vacuum delivery should be avoided as they have been shown to be associated with increased risk of MTCT. I f it has to be done, a vacuum with silastic cup is preferred
- Vaginal cleansing with chlorhexidine (0.25% solution) is believed to reduce the risk of puerperal and neonatal sepsis. It may also have some effects on HIV transmission where membranes are ruptured for >4 h. After every vaginal examination, the birth canal is wiped with gauze or cotton wool, soaked in chlorhexidine solution. The number of vaginal examinations should be kept to a minimum
- Routine episiotomy has been shown to have no obstetric benefit; it should be used only for specific obstetric indications.
Best obstetric practices
There should be capacity to:
- Train and retrain health-care providers in safe delivery techniques and life-saving skills for mothers and infants
- Provide safe delivery kits and essential obstetric drugs
- Provide a safe delivery infrastructure with a safe water source, good drainage, electricity, delivery beds covered with waterproof material, antiseptics, gloves, and other materials required for a hygienic delivery environment
- Ensure a safe blood supply
- Provide community education about the importance of ANC and hospital delivery.
Benefits of prevention of mother-to-child transmission
In settings where there are no PMTCTs or pediatric ART services, most children acquiring HIV through MTCT will die early in life (50% by their 2 nd year). The increasing number of AIDS-related deaths in under-five across Sub-Saharan Africa and Nigeria, in particular, is threatening to reverse the gains made by child survival programs. The cost of care and support for HIV-infected children place heavy financial burden on families, communities, and the health-care system. PMTCT benefits the mother, infant, family, community, and the health system in the following ways.
Benefits to the mother
- Identifies HIV-positive mothers for targeted interventions to reduce risk of transmission of infection to their babies and to access treatment, care, and support services
- Promotes positive behavior change and reduces risk of HIV transmission,
- Increases use of dual protection methods of FP and STI prevention
- Helps clients plan for the future
- Promotes infant feeding support
- Promotes access to early preventive and medical care
- Helps personal and financial decision-making.
Benefits to the infant
- Prevents HIV transmission to infants
- Promotes early diagnosis and intervention for the HIV-exposed infants
- Improves child health and survival.
Benefits to the family
- Promotes communication between couples and testing of both partners
- Reduces the risk of sexual transmission to serodiscordant partners
- Provides opportunity for testing other family members
- Contributes to reduction of stigma and discrimination
- Provides infant feeding support.
Benefits to the community
- Promotes the understanding of the HIV and AIDS epidemic among those living with HIV and AIDS within the community
- Promotes uptake of risk reduction practices
- Promotes acceptance and uptake of HIV testing and counseling
- Contributes to reduction of stigma and discrimination
- Provides infant feeding support.
Benefits to the health system
- Decreases the disease burden on the health system
- Gives an opportunity to strengthen the health system.
| Chapter 6: Use of Antiretroviral Drugs For Prevention Of Mother-To-Child Transmission | |  |
Overview
There is a significant amount of evidence available on the effectiveness of ARV prophylaxis to prevent MTCT of HIV. The evidence indicates the benefits of starting ARV prophylaxis during early pregnancy and its extended use for mothers or infants to prevent postpartum transmission through breastfeeding. Pregnancy constitutes an indication for the use of triple ARVs for therapy or prophylaxis.
ARV used in the Nigerian PMTCT program has evolved from single-dose nevirapine (NVP) in labor in 2003 (National PMTCT Guidelines, 2003) to triple ARV prophylaxis (tARVp) in 2010 (National PMTCT Guidelines, 2010). The 2010 National Guidelines recommends breastfeeding with tARVp coverage, which is stopped when the risk of MTCT ceases at the end of breastfeeding.
Eligibility criteria for antiretroviral prophylaxis
Pregnancy in the HIV-positive woman is an indication for tARVp irrespective of CD4, VL, or clinical stage. ARVs should be provided as soon as possible irrespective of gestational age. For positive pregnant women with CD4 count ≤500, these triple ARV regimens should be provided for life.
Antiretroviral therapy prophylaxis for prevention of mother-to-child transmission and duration of therapy
All pregnant and breastfeeding women with HIV should initiate triple ARVs (ART), which should be maintained at least for the duration of MTCT risk. Women meeting treatment eligibility criteria should continue lifelong ART. For women who are not eligible for ART for their own health, stop the ARV regimen 1 week after cessation of breastfeeding.
A once-daily fixed-dose combination of TDF + 3TC (or FTC) + EFV is recommended as the first-line ART in pregnant and breastfeeding women, including pregnant women in the first trimester of pregnancy and women of childbearing age [Table 4].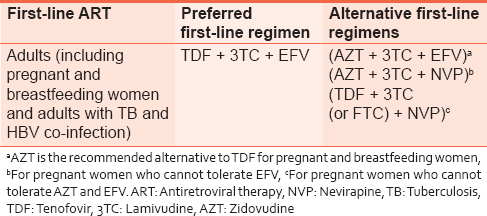 | Table 4: Recommended first - line antiretroviral regimen for use as fixed-dose combination tablet
Click here to view |
Infant feeding in the context of HIV
Introduction
Nutrition is an essential component of child-survival strategies. Optimizing infant and child nutrition is necessary to ensure good health and normal growth/development. Malnutrition is a common condition in HIV-infected children and is a major contributor to morbidity and mortality in this population. HIV infection can result in nutritional deficiencies, growth failure, and developmental delay. Malnutrition itself results in decreased immune function and greater susceptibility to infections, thus accelerating disease progression. Malnutrition makes HIV infection worse and HIV infection worsens malnutrition.
Goals of nutritional management for HIV-exposed and HIV-infected children
- Provide nutritional counseling to caregivers
- Provide nutritional care for children to maintain normal growth and development by:
- Encouraging exclusive breastfeeding for infants <6 months
- Introduction of complementary foods in infants beginning at 6 months
- Stopping breastfeeding at the age of 12 months or earlier when appropriate
- Maintaining adequate nutritional support during periods of illness.
Breastfeeding and HIV infection
Breastfeeding is one of the most important child-survival strategies. However, HIV can be transmitted through breast milk. Exclusive breastfeeding from birth in the presence of ARVs contributes to HIV-free survival in exposed infants. This avoids the risks and complexities associated with replacement feeding.
It is recommended that health-care providers counsel and support HIV-positive mothers to breastfeed their infants. Both mother and baby must however receive ARV drugs for prophylaxis or treatment as appropriate. This strategy will give Nigerian infants the greatest chance of HIV-free survival. However, women who choose not to breastfeed and those with medical contraindications to breastfeeding should be counseled and supported in their decision.
Complementary feeding
After 6 months of age, breast milk alone is not adequate for nutritional support of the infant. Complementary food should therefore be introduced for additional caloric requirements. The use of locally available foods is encouraged and is cheap and sustainable.
Examples of locally available complementary foods:
- Fruits, for example, bananas, plantains and cooked carrots
- Soft-prepared foods e.g. bean cakes (akara/kose), fortified pap (with palm oil, crayfish, groundnut), "Acha," "Tom Brown"
- High-protein foods: Beans, eggs, moin-moin, fish, and liver
- Green leafy foods, for example, vegetable soup.
When to stop breastfeeding
HIV-infected women who decide to stop breastfeeding should gradually wean over 1 month. Mothers or infants on ARV prophylaxis should continue prophylaxis for 1 week after complete cessation of breastfeeding. At this time, infants should be provided with safe and adequate complementary feeds to enable normal growth and development.
Growth monitoring and nutritional assessment
Regular and careful assessment of a child's growth helps monitor HIV disease progression, identify complications early, and offer the opportunity to intervene. Growth faltering may occur even before the emergence of opportunistic infections or other symptoms.
Growth monitoring include
History
- Feeding history (types and amounts of food taken, frequency of meals, problems with feeding)
- Potential causes of malnutrition (food insecurity, change of caregivers, illness)
- Assess for any major change in the child's circumstances
- Checking mother's health (need for ART) and her care of other children.
Anthropometry
- Measuring the weight, height, or length at every encounter with child and comparing with the age and sex-appropriate WHO Z-Score Card
- Measurement of mid-upper arm circumference and head (occipitofrontal) circumferences as indicated
- Using the Modified Wellcome Classification chart or other growth charts.
Complete physical examination and appropriate laboratory assessments should be performed.
Micronutrient supplements
All HIV-infected children should receive micronutrients as follows:
1. Single-dose Vitamin A supplementation every 6 months between 6 and 59 months of age as per the guidelines for uninfected children:
- <6 months - 50,000 IU
- 6-12 months - 100,000 IU
- 12 months-5 years - 200,000 IU.
2. Zinc supplements:
- <2 years 10 mg o.d. ×14 days
- >2 years 20 mg o.d. ×14 days.
3. Iron supplements:
- 3-6 mg/kg/day as required.
4. Folate supplements:
- <4 months 2.5 mg/day
- >4 months 5 mg/day.
5. Multivitamins, B6, B12, C, E.
Note: De-worming: Albendazole oral, 400 mg single dose every 6 months after the 1 st year of life.
Antiretroviral prophylaxis for HIV-exposed infants
All babies born to HIV-positive mothers are exposed to infection and should receive postexposure prophylaxis [Table 5].
Special situations for extended infant nevirapine prophylaxis
1. Mother is breastfeeding but not on HAART:
- Give daily NVP to infants from birth until 1 week after cessation of all exposure to breast milk.
2. Mother is breastfeeding and eventually commenced on HAART:
- Give daily NVP to infants from birth and continue until 12 weeks after maternal commencement of HAART.
Opportunistic infection prophylaxis for children
HIV-exposed infants should be offered CTX from age 6 weeks until HIV status is determined. If the HIV status is positive, CTX should continue; however, if negative, it should be stopped. CTX is given once daily. The recommended doses are shown in [Table 6] and [Table 7].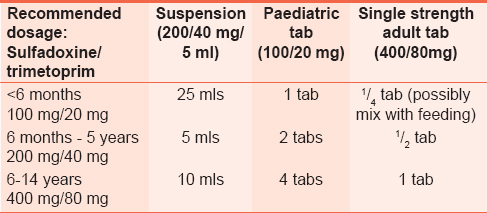 | Table 6: Dosing for co - trimoxazole prophylaxis in HIV - exposed infants and HIV - infected children
Click here to view |
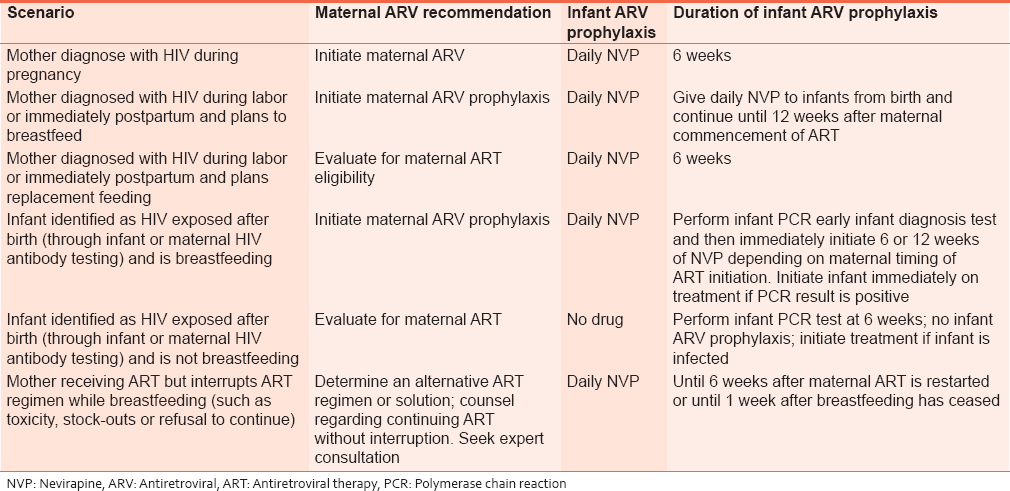 | Table 7: Summary of maternal and infant prophylaxis for different clinical scenarios
Click here to view |
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| References | |  |
| 1. | Jennifer S. Read; Preventing mother to child transmission of HIV: The role of caesarean section. Sex Transm Infect 2000;76:231-2. doi: l0.1l36/sti. 76.4.231.  |
[Table 1], [Table 2], [Table 3], [Table 4], [Table 5], [Table 6], [Table 7]
|