|


 |
| SHORT TECHNIQUE |
|
| Year : 2011 | Volume
: 3
| Issue : 2 | Page : 87-90 |
|
|
A new system for neuronavigation and stereotactic biopsy pantograph stereotactic localization and guidance system
Saeid Abrishamkar1, Houshang Moin1, Mohammadreza Safavi2, Azim Honarmand2, Mahmood Hajibabaie1, Elham K Haghighi1, Salman Abbasifard1
1 Department of Neurosurgery and Intensive Care Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
2 Anesthesiology and Critical Care Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
| Date of Web Publication | 11-Feb-2012 |
Correspondence Address:
Mohammadreza Safavi
Anesthesiology and Critical Care Research Center, Isfahan University of Medical Sciences, Isfahan
Iran
 Source of Support: None, Conflict of Interest: None
DOI: 10.4103/2006-8808.92800

 Abstract Abstract | | |
Everyday, neurosurgeons face the problem of orientation within the brain but the advent of stereotactic surgery and neuronavigation have solved this problem. Frame-based stereotactic systems (FBSS) and neuronavigation systems have their own strengths and priority and pitfalls, which were the main driving force for us to design a new system. This hybrid system comprises three main parts: main frame, monitoring system, and pantograph, which are connected to each other and to the operating table by particular attachments. For using this system, after performing CT SCAN or Magnetic Resonance Imaging (MRI) the axial view will be transferred to Liquid Cristal Display (LCD). In the operating room, the head of the patient fixes to the operating table and registration is completed by two arms of pantograph. We made a simulation operation with our system on an occipital cavernous angioma and a frontal oligodendroglioma. The software, which have been used for simulation were as follows; Poser (version-7), Catia (version 5- R18), and 3 Dimension Max (version 2008). The accuracy of this system is approximately two millimeter. The advantages of this system are: easy to use, much less expensive, and compatible with different devices, which may be needed during neurosurgical operation. For countries that do not have the opportunity to have sophisticated technology and neuronavigation system, we believe that our system is a one-stop solution. Keywords: Neuronavigation, pantograph, stereotactic surgery
How to cite this article:
Abrishamkar S, Moin H, Safavi M, Honarmand A, Hajibabaie M, Haghighi EK, Abbasifard S. A new system for neuronavigation and stereotactic biopsy pantograph stereotactic localization and guidance system. J Surg Tech Case Report 2011;3:87-90 |
How to cite this URL:
Abrishamkar S, Moin H, Safavi M, Honarmand A, Hajibabaie M, Haghighi EK, Abbasifard S. A new system for neuronavigation and stereotactic biopsy pantograph stereotactic localization and guidance system. J Surg Tech Case Report [serial online] 2011 [cited 2016 Jun 12];3:87-90. Available from: http://www.jstcr.org/text.asp?2011/3/2/87/92800 |
 Introduction Introduction | |  |
Neurosurgeons daily face the problem of orientation within the brain. Stereotaxis as a concept in neurosurgery began by Horsley and Clarke in 1908. The practical application of stereotaxis waited until 1947, when Spiegel and Wycis began their pioneering work. Many giant neurosurgeons find different solution for neuronavigation and stereotactic surgery. [1],[2],[3],[4],[5] Soon then image-guided surgical navigation lead to the development of new methods of less invasive surgery of the nervous system. [4],[5] But still the major limitations related to preoperative data were, one; the three-dimensional (3D) data set has limited value unless the imaging coordinates in some way can be related to the absolute position of the patient's head and two; the images have limited or no value when the anatomy changes, for example during tumor resection or cyst/ventricle aspiration. [6],[7],[8] The first limitation has been partially solved by stereotactic devices and neuronavigation systems. For stereotactic systems, however, the equipment are cumbersome to handle, and the access to the lesion is limited to a single trajectory. [6],[7],[8] For frame-based stereotactic system, the main problem was that the neurosurgeons were not able to perform a craniotomy and access to pathology was possible only for biopsy or a very limited craniotomy. [5],[6],[7],[8],[9] On the other hand, for neuronavigation systems beside the brain shift, the other source of pitfalls (e.g. technical and registration errors) exists. [8],[9],[10] Considering all these advantages and disadvantages, we decided to design a hybrid system based on the strong points and priority of stereotactic and neuronavigation systems.
 Technique Technique | |  |
Pantograph Stereotactic Localization and Guidance System (PSLGS) consists of three main parts: (1)- Special pantograph,( 2)- Main frame, which supports the head of patient, and (3)- monitoring system, which is attached to the main frame and show the CTS or Magnetic Resonance Imaging (MRI) images [Figure 1] and [Figure 2]a, b, c and d. The main frame of system is fixed to the head of the patient by its three arms [Figure 1] and [Figure 2]a. The arms have been designed in a manner that they could rotate around the ring. Patient will transferred to imaging center and a CT Scan or MRI perform. The slices of images aggregate one or two millimeter apart according to request of neurosurgeon and the axial images will be transferred to Liquid Cristal Display (LCD). In the operating room, the main frame is fixed to operating table and LCD is attached to the main frame by its special arms [Figure 1] and [Figure 2] c and d. These arms are designed so that the LCD could be at any position in relation to the head of the patient. Four guideline points have been designed over the frame, which will be used to adjust the data of the patient image with their counterparts on LCD by pantograph. The pantograph, which is also attached to main frame has two arms and can easily move at any direction [Figure 1] and [Figure 2]e and f. The navigator part of the pantograph (the first arm) can support biopsy needle, CUSA, endoscope, or any other devices of operation. The other arm of pantograph has a pointer that move at the same direction and plane as the navigator arm displace. A special LASER pointer is located over this second arm of pantograph which shows the directions of pointer before it reach the surface of LCD [Figure 1] and [Figure 2] e, so this arm show the data over LCD and the other arm (navigator arm) show the counterpart data over the patient's brain and cranium [Figure 1] and [Figure 2]e and f. The arms of pantograph are free to move at any direction. We designed a simulation operation on two cases, an occipital cavernous angioma and a frontal oligodendroglioma. The thickness of slices for all images was two millimeter. The brain of both cases that has been seen on CTS and MRI were reconstructed and simulated and then a simulation films were constructed to see the ability of system to detect and localize the pathology. The software that have been used for reconstruction and simulation were; Poser (version 7), Catia (version 5- R18), and 3 Dimension Max (version 2008).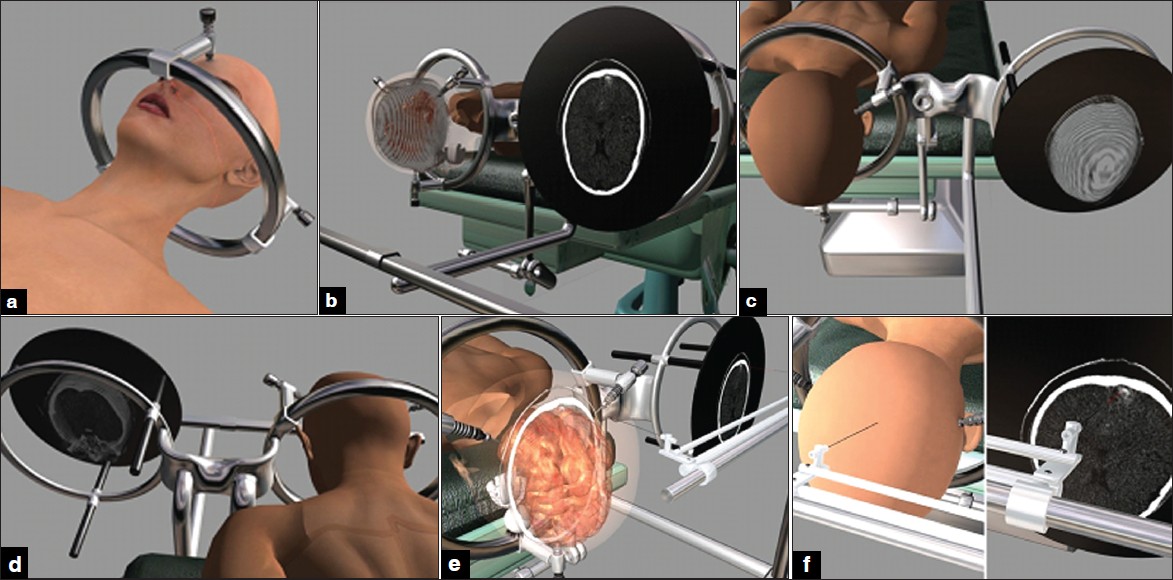 | Figure 1: An occipital cavernous angioma at the tip of left occipital lobe. (a) The frame has been fi xed to the cranium. (b) The head of the patient have been fi xed to operating table at prone position and the images have been transferred to monitor. (c and d) The patient and images data have been adjusted. (e and f) The pantoghraph show that the data of the patient head and pathology are seen at the same location of their counterpart on LCD.
Click here to view |
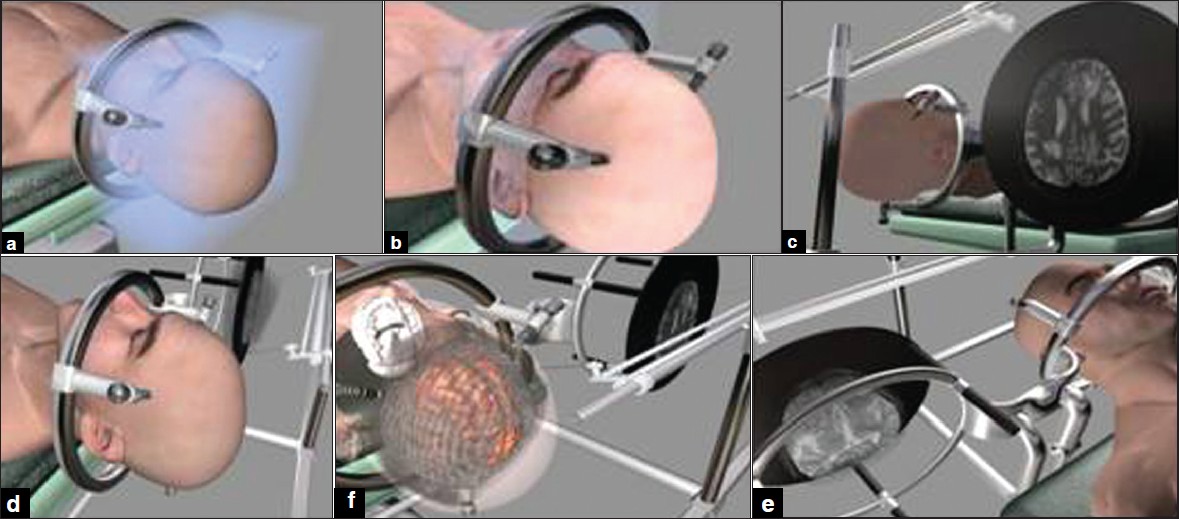 | Figure 2: A right frontal oligodendroglioma confi rmed by stereotactic biopsy. (a) The frame has been fi xed to the cranium. (b) The head of patient have been fi xed at supine position to operating table and the MRI images have been transferred to monitor. (c and d) The patient and images on LCD data have been adjusted. (e and f) The pantoghraph show that the data of the patient head and pathology are seen at the same location of their counterpart on LCD.
Click here to view |
 Results Results | |  |
We designed a simulation operation on two cases, an occipital cavernous angioma and a frontal oligodendroglioma. The first case was all data related to a female, 27-year-old with cavernous angioma, who was referred to us because of progressive homonymous hemianopia and visual loss. A simulation operation with the CTS (2 millimeter apart) was designed. The second patient was a 55-year-old male who suffered from oligodendroglioma. A simulation operation with MRI (T2 series) was designed and the capabilities of system evaluated. These two cases were selected because they were at opposite site and exactly at two extreme parts of the cranium and brain.
 Discussion Discussion | |  |
The main problem ahead on many neurosurgeons is the way they could approach a deep lesion inside cranial cavity [1],[2],[3],[4],[5] . although sterotactic and neuronavigation have their own benefits but have also pitfalls as well [10],[11],[12] . So in our hybrid system we decided to use the priority of each system. Frame-based stereotactic systems (FBSS) have the advantage of proven clinical utility and instrument carriage with a high degree of mechanical stability and accuracy on the other hand frameless methods are more complex, but also more flexible, and may have wide applications in general neurosurgery. [10],[11],[12],[13] In FBSS, neurosurgeons do not have any opportunity to change the trajectory or the plain of biopsy during operation but, with PSLGS it is easy to choice or change the new target on CTS or MRI throughout the operation like any other neuronavigation systems. [14],[15] For some systems, such as Sonowand, the possibility of using intra-operative sonoghraphy during operation exists, but this advantage needs a lot of equipments and technology that also could not be fined for many neurosurgical centers. For FBSS, the frame and the arc of the system is a great barrier for neurosurgeon to perform a craniotomy; however, in neuronavigation system this is not the case. In our system, the frame is so designed that the pedicles can rotate around the main frame and provides a very large field of operation or craniotomy. On the other hand, for Lekcell and other stereotactic system the manipulation of arc and other compartments of systems is not so easy and may change the data of registration during operation but with PSLGS the neurosurgeon does not need to use the three-point cranial holder (e.g. Mayfield) before operation as it should be considered for neuronavigaiton system. Except for the needle of biopsy, other devices are not compatible with stereotactic systems. Our system is so designed that it can support all devices that neurosurgeon need during operation such as CUSA, suction tube, sonoghraphy probe and so on. This is one of the most important neuronavigaiton system priorities that we have considered for our system as well. In PSLGS all of the devices can be hold by the arm of pantograph and could be fixed at any position during operation. The registration is mechanical in PSLGS so the chance of human error is very low as it may occur for some of neuronavigation system. The first plane of images is at the same plane of monitor (LCD), which is at its default position. The only registration that neurosurgeons have to do before operation is to adjust one of the predicted markers over the frame with its counterpart on LCD slices but for neuronavigation system it takes at least half an hour for image and patient registration. Finally, PSLGS is easy to use and less expensive. For countries that do not have the chance of having sophisticated technology and neuronavigation, we believe that our system can provide one of the best solutions.
 References References | |  |
| 1. | Gildenberg PL, Kaufman HH, Murthy KS. Calculation of stereotactic coordinates from the computed tomographic scan. Neurosurgery 1982;10:580-6. 
[PUBMED] |
| 2. | Dietrichs E. Movement disorders and basal ganglia function. Tidsskr Nor Laegeforen 2008;128:1968-71. 
[PUBMED] [FULLTEXT] |
| 3. | Abosch A, Lozano A. Stereotactic neurosurgery for movement disorders. Can J Neurol Sci 2003;30:S72-82. 
[PUBMED] |
| 4. | Tubbs RS, Hill M, Loukas M, Shoja MM, Oakes WJ. Volumetric analysis of the posterior cranial fossa in a family with four generations of the Chiari malformation Type I. J Neurosurg Pediatr 2008;1:21-4. 
[PUBMED] [FULLTEXT] |
| 5. | Nair DR, Burgess R, McIntyre CC, Lüders H. Chronic subdural electrodes in the management of epilepsy. Clin Neurophysiol 2008;119:11-28. 
|
| 6. | Apuzzo ML, Chandrasoma PT, Cohen D, Zee CS, Zelman V. Computed imaging stereotaxy: Experience and perspective related to 500 procedures applied to brain masses. Neurosurgery 1987;20:930-7. 
[PUBMED] |
| 7. | Laitinen LV. My 50 years of interest in stereotactic and functional neurosurgery. Stereotact Funct Neurosurg 2001;77:7-10. 
[PUBMED] [FULLTEXT] |
| 8. | Johnson RD, Stacey RJ. The impact of new imaging technologies in neurosurgery. Surgeon 2008;6:344-9. 
[PUBMED] |
| 9. | Grunert P, Darabi K, Espinosa J, Filippi R. Computer-aided navigation in neurosurgery. Neurosurg Rev 2003;26:73-99. 
[PUBMED] |
| 10. | Greenberg MM, Dekel D, Zinreich SJ, Bryan RN. Probe-correlated viewing of anatomical image data. Patent No. CA-003497, 1989. 
|
| 11. | Kalfas IH. Image-guided spinal navigation. Clin Neurosurg 2000;46:70-88. 
[PUBMED] |
| 12. | Tuominen J, Yrjänä SK, Katisko JP, Heikkilä J, Koivukangas J. Intraoperative imaging in a comprehensive neuronavigation environment for minimally invasive brain tumour surgery. Acta Neurochir Suppl 2003;85:115-20. 
|
| 13. | Zamorano L, Kadi AM, Dong A. Computer-assisted neurosurgery: Simulation and automation. Stereotact Funct Neurosurg 1992;59:115-22. 
[PUBMED] |
| 14. | Dorward NL, Paleologos TS, Alberti O, Thomas DG. The advantages of frameless stereotactic biopsy over frame-based biopsy. Br J Neurosurg 2002;16:110-8. 
[PUBMED] |
| 15. | Abrishamkar S, Aminmansour B, Arti H. The effectiveness of computed tomography scans versus magnetic resonance imaging for decision making in patients with low back pain and radicular leg pain. J Res Med Sci 2006;11:351-4. 
|
[Figure 1], [Figure 2]
|