|


 |
| CASE REPORT |
|
| Year : 2012 | Volume
: 4
| Issue : 1 | Page : 39-42 |
|
|
Coexistent malignant peripheral nerve sheath tumor and lateral spinal meningoceles
Lata Bhoir, Pramod Nichat, Ashish Chug, Harish Verma
Department of Surgery, B. J. Medical College and Sassoon General Hospitals, Pune, Maharashtra, India
| Date of Web Publication | 5-Sep-2012 |
Correspondence Address:
Lata Bhoir
2N Lotus B, Flat No. 16, Aditya's - A Garden City, Warje, Pune - 411 058, Maharashtra
India
 Source of Support: None, Conflict of Interest: None
DOI: 10.4103/2006-8808.100353

 Abstract Abstract | | |
Malignant peripheral nerve sheath tumor (MPNST) is a malignant spindle cell tumor of the soft tissue thought to be derived from the components of nerve sheath. MPNSTs are mainly located in the buttocks, thighs, brachial plexus, and paraspinal region. The objective of this article is to describe a case of neurofibromatosis type 1 who developed neurofibrosarcoma of the right lateral thoracic nerve with thoracic meningoceles, a rare coincidental finding which has not yet been reported in the English medical literature, and how both the conditions were managed in the same sitting. Keywords: Lateral thoracic meningocele, malignant peripheral nerve sheath tumor, Neurofibromatosis type 1, soft tissue sarcoma, thoracotomy
How to cite this article:
Bhoir L, Nichat P, Chug A, Verma H. Coexistent malignant peripheral nerve sheath tumor and lateral spinal meningoceles. J Surg Tech Case Report 2012;4:39-42 |
How to cite this URL:
Bhoir L, Nichat P, Chug A, Verma H. Coexistent malignant peripheral nerve sheath tumor and lateral spinal meningoceles. J Surg Tech Case Report [serial online] 2012 [cited 2016 Jun 10];4:39-42. Available from: http://www.jstcr.org/text.asp?2012/4/1/39/100353 |
 Introduction Introduction | |  |
Malignant peripheral nerve sheath tumor (MPNST) is a malignant spindle cell tumor of the soft tissue thought to be derived from the components of nerve sheath such as perineural fibroblasts or Schwann cells. [1] These tumors are treated as a subcategory of soft tissue sarcoma, in which they comprise 3-10% of all such tumors. [1] MPNSTs are rare tumors with an incidence of 0.001% in the general population. [2] Till date,very few cases of MPNSTs of the lateral thoracic nerve have been reported in the medical literature. [3] Coincidental meningoceles are further rare.
 Case Report Case Report | |  |
A 60-year-old male, a case of neurofibromatosis type 1, presented with swelling in the right axilla, with a rapid growth over 5-6 months. The patient had difficulty in raising the right upper limb above shoulders. He also had breathlessness on exertion.
There was no history of trauma or tingling or numbness.
On examination, there was 14Χ11 cm size swelling over the right lateral aspect of the thorax extending into the axilla, with the presence of dilated veins over it. The local temperature was normal. It was freely mobile over underlying structures. The overlying skin was nonadherent. There was winging of the scapula indicating the involvement of the lateral thoracic nerve [Figure 1]a.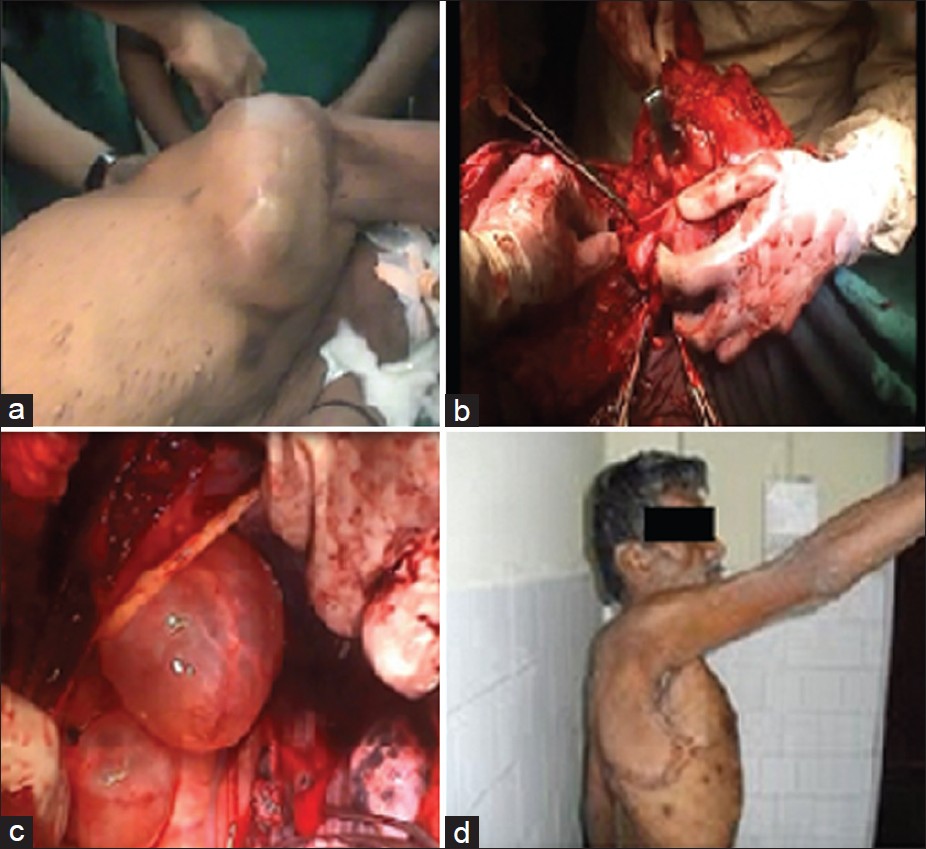 | Figure 1: (a) Preoperative photo of the patient. (b) Photo showing
tumor arising from the lateral thoracic nerve. (c) Photo showing lateral
thoracic meningoceles. (d) Postoperative photo of the patient
Click here to view |
The PA view of the chest X-ray showed a space-occupying lesion on the right side paraspinally extending from D2 to D7 [Figure 2].
A CT scan of the thorax showed a mixed density mass of 14Χ11 cm arising from the neurovascular bundle with a heterogeneous enhancement on the postcontrast study. There was no evidence of calcification. A cystic lesion of about 8Χ5 cm size was also seen in the right intrathoracic paraspinal region [Figure 3]a and c.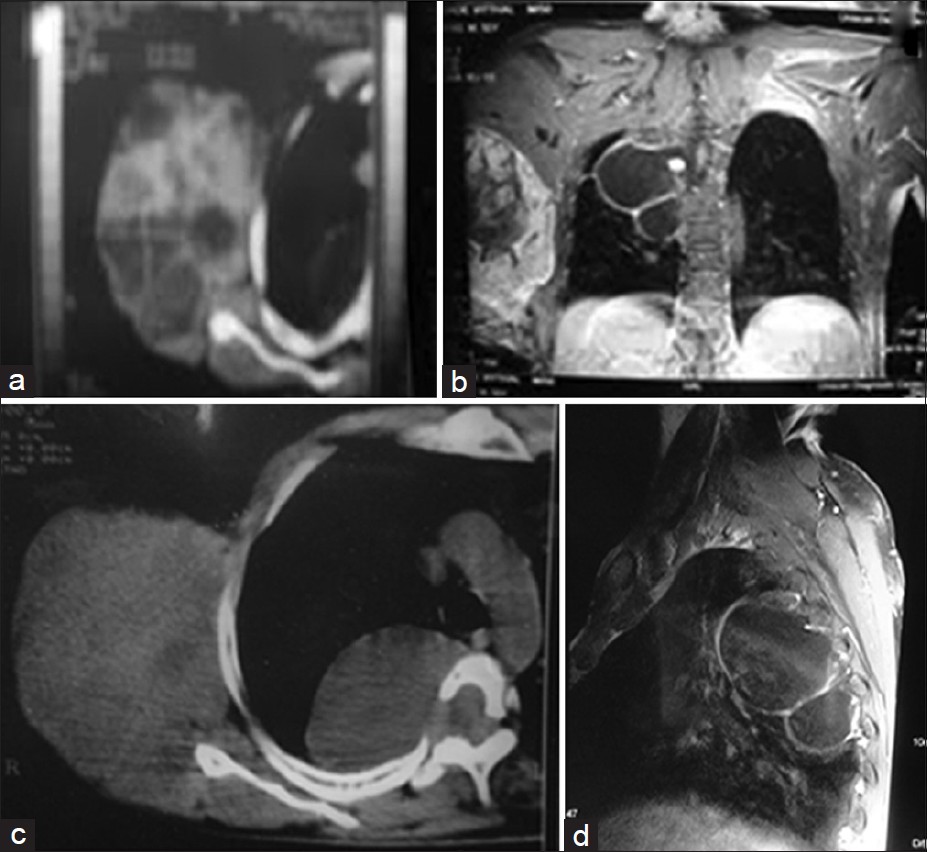 | Figure 3: (a) CT scan of the tumor. (b) MRI showing both the tumor
and the meningoceles. (c) CT scan showing lateral meningocele
with a spinal defect and the tumor. (d) MRI lateral view showing the
meningoceles
Click here to view |
As the nature of the intrathoracic paraspinal swelling was doubtful, magnetic resonance imaging (MRI) was done.
MRI revealed widened D1-8 neural foramina on the right side with widened subarachnoid spaces. There was evidence of T2 hyperintense space-occupying lesions in the right paravertebral region from D1-2 to D7-8 levels which appeared lobulated and septated with well-defined walls, contiguous with each other, and extending into the spinal canal via large neural foramina. There was no internal enhancement on the postcontrast study suggestive of thoracic meningoceles/duralectasia [Figure 3]b and d.
Tru-cut biopsy of the swelling indicated malignancy.
Surgical technique
In view of the presence of the lesions on the right side and history of breathlessness, a decision was taken to operate both the pathologies in the same sitting.
Under general anesthesia,the patient was given a left lateral position with hyperabducted right arm.An elliptical incision was taken on the posterior axillary fold so as to remove the redundant skin along with the mass.
Procedure
Subcutaneous flaps were raised and a wide (5 cm from all sides) local excision was done. During dissection, it was found that the tumor was arising from the right lateral thoracic nerve. The nerve along with the mass was excised [Figure 1]b and [Figure 4]a & b.
After the complete removal of the tumor, a right lateral thoracotomy was done to repair the meningoceles in the same position. The meningoceles were dissected till their openings into the defects and then the defects were repaired with a G-patch (artificial dural graft; [Figure 1]c and [Figure 4]c).
Intercostal and subcutaneous suction drains were kept before closure. The patient recovered uneventfully.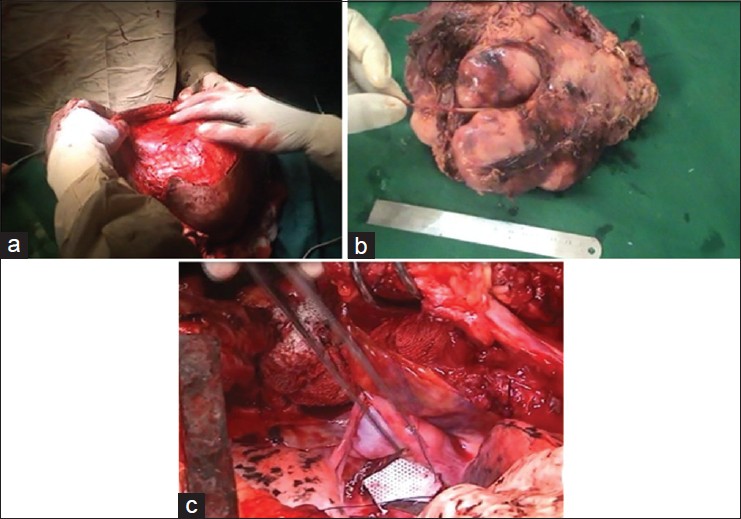 | Figure 4: (a) Intraoperative photo while raising skin flaps. (b) Tumor
with the lateral thoracic nerve excised in TOTO. (c) Repair of
meningocele defects being done with a G‑patch (artificial dural patch)
Click here to view |
The histopathology of the tumor suggested malignant spindle cell neoplasm, with areas of necrosis, hyalinization, and myxoid change with giant cell reaction to the tumor [Figure 5]a and b.
Immunohistochemistry (IHC) suggested malignant spindle cell neoplasm positive for vimentin with negativity for S100 indicating decreasing differentiation. Diagnosis of the MPNST was confirmed [Figure 6].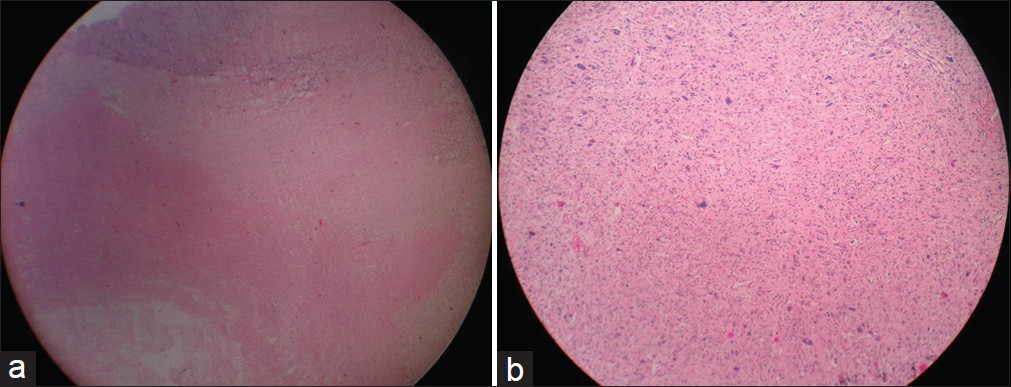 | Figure 5: (a) Histopathology scanner view (×4) showing tumor with
areas of necrosis. (b) Histopathology photographic view (×10) showing
tumor cells arranged in fascicles with nuclear pleomorphism
Click here to view |
In view of the likely residual microscopic disease, the patient was given adjuvant radiotherapy (RT).
On follow-up at 6 months, the patient did not have local recurrence [Figure 1]d.
 Discussion Discussion | |  |
The incidence of neurofibromatosis is about 1/3000 of the population. [4],[5]
The disease is characterized by a classical triad of skin lesions, mental deficiency, and skeletal deformities. It was described for the first time by Friedrieh Daniel von Recklinghausen in 1882. [6] Now we distinguish neurofibromatosis as types 1 and 2 (NF 1 and NF 2), which are the peripheral and the central forms of the disease, respectively. [5],[7]
As defined by the WHO, MPNSTs are malignant tumors arising from a peripheral nerve or showing a nerve sheath differentiation with the exception of tumors originating from the epineurium or the peripheral nerve vasculature. [8] Approximately, one-third of MPNSTs arise de novo, whereas the remainder represent the sarcomatous degeneration of a preexisting plexiform neurofibroma.
MPNSTs are associated with genetic aberrations such as loss of the neurofibromatosis 1 gene protein product. MPNSTs occur in the third to sixth decade of life usually arising from medium and larger nerves. Tumor location has been found to be a strong prognostic factor, with those in the thoracic and retroperitoneum having worse outcomes. [2] In our patient, the MPNST was found to be arising from the right lateral thoracic nerve, suggesting a better prognosis with good surgical resection.
The International Consensus Group has recommended that the current management of MPNSTs should be identical to that of any other soft tissue sarcomas (STSs),. Adjuvant RT should be considered for all intermediate- and high-grade lesions as well as low-grade tumors with positive margins. [9] Our patient also received RT as the tumor was negative for S100 which indicates decreasing differentiation. MPNSTs are more aggressive tumors compared with other STSs; hence, an attempt should be made for near total surgical debulking of the tumor.
The supportive literature is generally in the context of sarcomas and is not specific to MPNSTs. The role of chemotherapy is limited to the treatment of metastatic disease. Local recurrences have been reported to vary from 52 to 88.9% for different sites. Metastasis, in the lungs and liver, ranged from 11.1 to 18%. [10] Disease-free survival and overall survival were reported to be approximately 64% and 30% at 5 years, respectively.
The etiology of meningoceles in NF 1 remains controversial. The changes in neurofibromatosis can be classified as primary, which are related to mesodermal dysplasia; secondary, which result from the compressive effects of nerve sheath tumors; and mixed that combine the two previous. Ventrolateral meningoceles of the thoracic spine are secondary to congenital mesodermal dysplasia and hypoplastic bone changes. [11] Our patient had the meningoceles since a long time, but presented with symptoms like breathlessness along with the MPNST. We postulate that the MPNST arose in a preexisting neurofibroma in this patient of NF 1 who already was harboring lateral thoracic meningoceles. Such a case of co-existent meningoceles and MPNST has not been reported yet in any English medical literature.
 Conclusion Conclusion | |  |
Rarity of this tumor along with lateral spinal meningoceles made us to present this case with a multidisciplinary approach to tumor still undefined.
 References References | |  |
| 1. | Al-Gahtamy M, Midha R, Guha A, Jacobs WB.Malignant peripheral nerve tumors.In:Beyer MS, Prados MD, editors.Textbook of Neuro-oncology. Philadelphia:Elsevier Saunders;2005.p.564-71. 
|
| 2. | Wanebo JE, Malik JM, VandenBerg SR, Wanebo HJ, Driesen N, Persing JA.Malignant peripheral nerve sheath tumors. A clinicopathological study of 28 cases. Cancer 1993;71:1247-53. 
[PUBMED] |
| 3. | George E, Swanson PE, Wick MR. Malignant peripheral nerve sheath tumorsof the skin. Am J Dermatopathol1989;11:213-21. 
[PUBMED] |
| 4. | Erkulvrawatr S, El Gammai T, Hawkins J, Green JB, Striniyasan G. Intrathoracicmeningocelesand neurofibromatosis. Arch Neurol 1979;36:557-9. 
|
| 5. | Freund B, TimonC.Cervical meningocele presenting as a neck mass in a patient with neurofibromatosis 1. J LaryngolOtol1992;106:463-4. 
|
| 6. | DolynchukKN, TeskeyJ, West M.Intrathoracicmeningocele associated with neurofibromatosis: Case report. Neurosurgery 1990;27:485-7. 
|
| 7. | O'neill P, Whatmore WJ, Booth AE.Spinal meningoceles in association with neurofibromatosis. Neurosurgery 1983;13:82-4. 
[PUBMED] |
| 8. | World Health Organization.Pathology and Genetics of Tumors of the NervousSystem. Lyon:IARC Press;2000.p. 169-71. 
|
| 9. | Ferner RE, Gutmann DH.International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res 2002;62:1573-7. 
[PUBMED] |
| 10. | Vege DS, Chinoy RF, Ganesh B, Parikh DM.Malignant peripheral nerve sheath tumors of the head and neck: A clinicopathological study. J SurgOncol1994;55:100-10. 
[PUBMED] |
| 11. | Rainov NG, Heidecke V,Burkert W. Thoracic and lumbar meningocele in neurofibromatosis type 1. Report of two cases and review of the literature. Neurosurg Rev 1995;18:127-34. 
[PUBMED] |
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5], [Figure 6]
|