|


 |
| CASE REPORT |
|
| Year : 2012 | Volume
: 4
| Issue : 1 | Page : 53-57 |
|
|
Direct, high-flow bypass for a pediatric giant, fusiform aneurysm of the inferior division of m2: Case report and review of literature
Vignesh K Alamanda, Luke Tomycz, Dennis Velez, Robert J Singer
Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN, USA
| Date of Web Publication | 5-Sep-2012 |
Correspondence Address:
Robert J Singer
Department of Neurological Surgery, Vanderbilt University Medical Center, T 4224 Medical Center North, Nashville, Tennessee 37232 2380
USA
 Source of Support: None, Conflict of Interest: None
DOI: 10.4103/2006-8808.100357

 Abstract Abstract | | |
In this case report, we describe the first reported case of treating a 7-year-old male patient who has a giant, fusiform aneurysm confined to the inferior M2 segment by means of a saphenous vein graft. Given the lack of good endovascular management options for this particular scenario, craniotomy was recommended and an end-to-side ECA-ICA anastomosis was carried out with technical details of the surgery outlined in the manuscript. The patient did not sustain any major postoperative complications. The graft remained patent upon completion of the surgery and at the time of last follow-up, 9 months post-surgery. This case serves as an illustrative example of the need for high-flow bypass for a select few patients even as endovascular technology continues to improve. Keywords: Aneurysm, bypass, fusiform, giant, high flow, inferior M2 division
How to cite this article:
Alamanda VK, Tomycz L, Velez D, Singer RJ. Direct, high-flow bypass for a pediatric giant, fusiform aneurysm of the inferior division of m2: Case report and review of literature. J Surg Tech Case Report 2012;4:53-7 |
How to cite this URL:
Alamanda VK, Tomycz L, Velez D, Singer RJ. Direct, high-flow bypass for a pediatric giant, fusiform aneurysm of the inferior division of m2: Case report and review of literature. J Surg Tech Case Report [serial online] 2012 [cited 2016 Jun 10];4:53-7. Available from: http://www.jstcr.org/text.asp?2012/4/1/53/100357 |
 Introduction Introduction | |  |
Cerebral aneurysms rarely occur in the pediatric population with a reported prevalence ranging from 0.5 to 4.6%, and are clinically, morphologically, and pathologically distinct from their adult counterparts. [1] Based on prior series and case reports, pediatric intracranial aneurysms are more likely to present with neurological signs and symptoms of mass effect as opposed to subarachnoid hemorrhage. There seems to be a male predominance (1.8:1) and preferential location within the posterior circulation or at the ICA bifurcation. Furthermore, compared to aneurysms in adults, a considerable proportion of pediatric aneurysms is giant, dissecting, or fusiform, and thus may be less amenable to endovascular treatment. [2] Complex microsurgical solutions including clip reconstruction, trapping, and high-flow bypass may often provide the most durable treatment option for these challenging lesions.
 Case Report Case Report | |  |
We present the case of a 7-year-old male presenting with chronic headache and an unruptured, giant/fusiform intracranial aneurysm. To our knowledge, this is the first reported case of a giant, fusiform aneurysm in a child, confined to the inferior M2 segment, and treated with high-flow, saphenous vein bypass. Special care is taken to highlight nuances of the operative technique aimed at complication avoidance.
Presentation and history
The patient presented to an outside hospital with a complaint of chronic headache. MRI/MRA revealed a large aneurysm of the right middle cerebral artery (MCA) bifurcation. Past medical history was significant for premature birth, developmental delay, asthma, ADHD, and a recent diagnosis of "mild cerebral palsy".
At presentation to our center, the patient was examined and found to be neurologically intact. Subsequent digital subtraction cerebral angiography demonstrated a giant, fusiform (29×17 mm) aneurysm involving the inferior division of the right M2 segment After extensive counseling with the patients family, craniotomy was recommended and scheduled [Figure 1].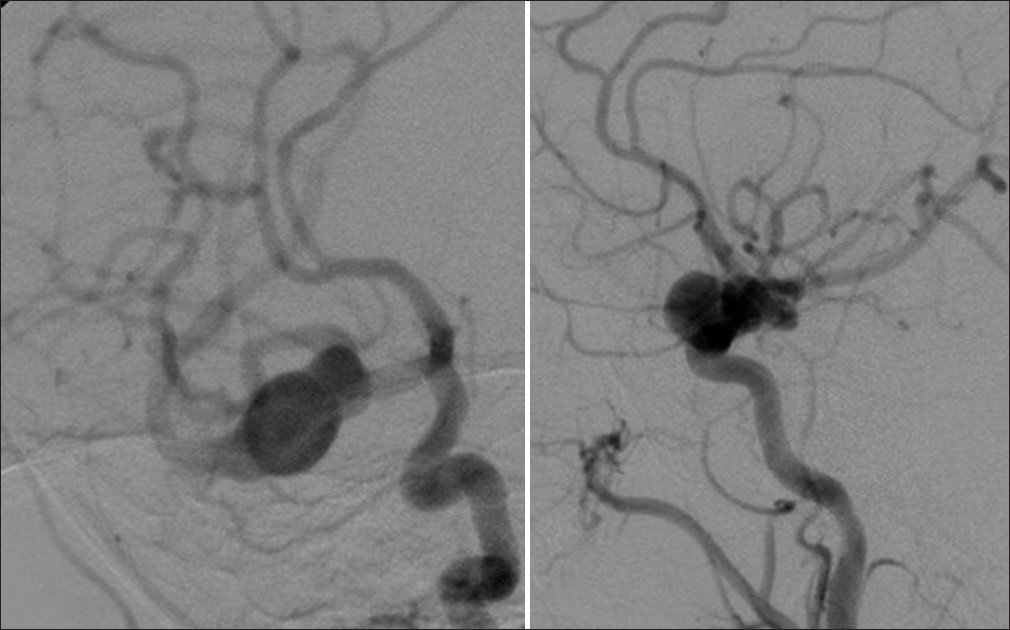 | Figure 1: AP and lateral view, respectively, of the giant fusiform aneurysm
Click here to view |
Surgical technique
A standard right pterional craniotomy with Sylvian dissection was performed to expose the middle cerebral tree and aneurysm at the inferior division of the right M2 segment.
Harvesting of the saphenous vein graft
A 3 cm incision was made anterior to the medial malleolus on the left leg with a #15 blade scalpel. The saphenous vein was then carefully exposed and the associated branches were divided using a 3-0 silk tie on the graft side and small clips on the distal component. A 25 cm segment of saphenous vein was isolated and divided both at the proximal and distal extent with 4-0 silk ties. Using an awl-tipped syringe secured into the distal end of the graft and bulldog vascular clamps proximally, the graft was flushed and dilated throughout its length. There was one small side branch that needed to be ligated with a 6-0 prolene in a figure-of-eight manner. This was then placed in normal saline solution until the surgeons were ready to sew in the graft for by-pass.
Cervical carotid exposure and craniotomy
A linear incision was made along the medial border of the right sternocleidomastoid. Following separation of the platysma, the carotid sheath was bluntly dissected, and the proximal ICA and ECA were identified and isolated at the common carotid bifurcation. Vessels loops were placed around the isolated segments to facilitate manipulation of the exposed branches in anticipation of ECA trapping for proximal anastomosis.
A traditional right-sided pterional craniotomy was performed with wide splitting of the Sylvian fissure and extensive drilling of the sphenoid wing, effectively exposing the supraclinoid carotid and the proximal portion of the right M1 and A1 segments. The M1 was followed distally to the MCA bifurcation and beyond to the aneurysmal dilation of the inferior M2 division. Adequate exposure for proximal and distal control was achieved with the ultimate intention of trapping the fusiform segment [Figure 2].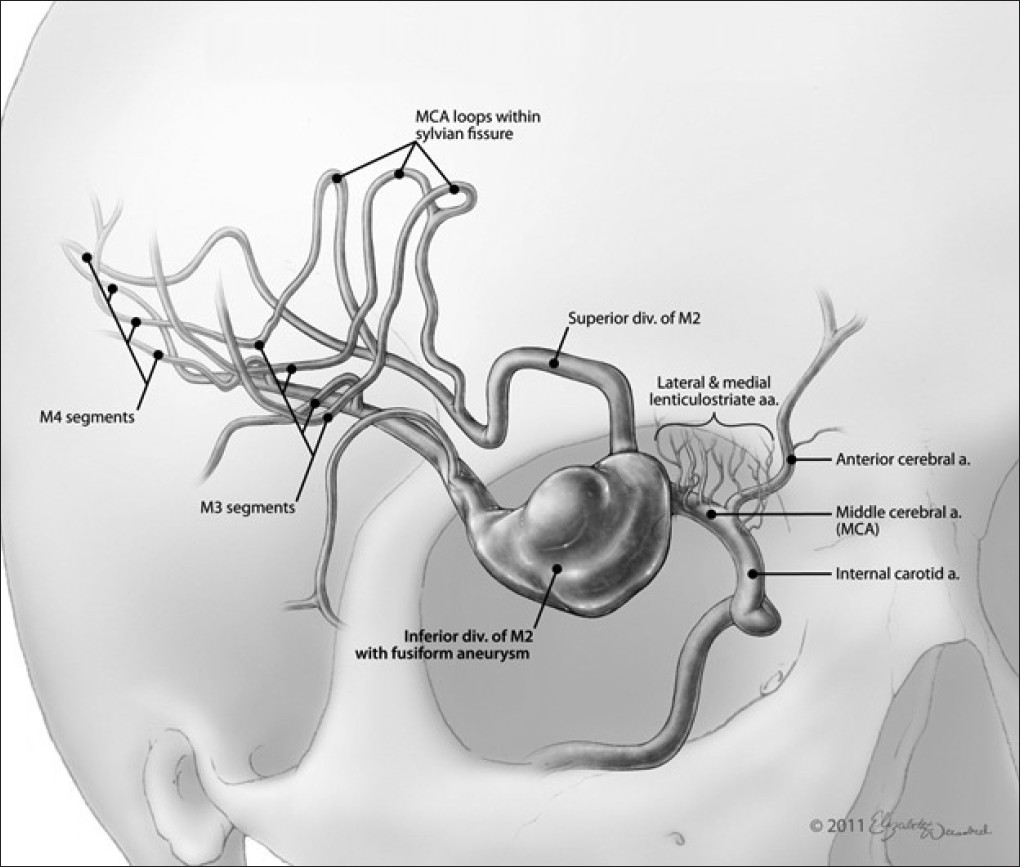 | Figure 2: Artistic rendition of the giant fusiform aneurysm of the inferior M2 division
Click here to view |
Proximal and distal anastamosis
A suitable recipient site for the saphenous vein graft was isolated within the proximal ECA. Then, with sequential dilation, a tract was placed anteriorly to the tragus for the passage of the saphenous vein graft. The graft was then tunneled through an endotracheal tube from proximal to distal in anticipation of anastomosis. The saphenous vein adventitia was denuded proximally and cut in a fishmouth fashion with microscissors. Meanwhile, aneurysm clips were placed on the proximal external carotid artery to trap a segment for anastomosis. The trapped segment was then opened with a microknife. Using a combination of interrupted and running suture techniques, #8-0 prolene was used to anastomose the proximal end of the graft to the trapped segment of ECA in an end-to-side fashion. Graft patency and competency of the suture line were ensured after completion of the anastomosis. A clip was placed on the proximal segment of the graft.
Attention was then shifted to the intracranial compartment. With deepening subfrontal retraction and induction of burst-suppression along with gentle hypertension, a temporary clip was placed on the distal right M1 segment. Full heparinization was instituted with the initiation of temporary clipping. This maneuver enabled us to more safely manipulate the aneurysm and clearly delineate the proximal and distal aspects of the fusiform dilation as well as identify adjacent perforators. Ten-millimeter permanent aneurysm clips were then placed so as to trap the fusiform segment of the inferior M2 division. Once the aneurysm was effectively trapped, the temporary clip on the M1 segment was removed restoring blood flow to the unaffected superior M2 division.
An additional temporary clip was placed further distally along the M2 division to trap the recipient site for distal anastomosis. The distal graft was once again cut in a fishmouth fashion. A combination of running and interrupted #9-0 proline sutures was then used to complete the distal anastomosis. Graft patency was ensured and all temporary clips were removed. Flow within the pulsatile graft was confirmed with a micro-Doppler probe. Intraoperative angiogram confirmed exclusion of the aneurysm and patency of the graft [Figure 3].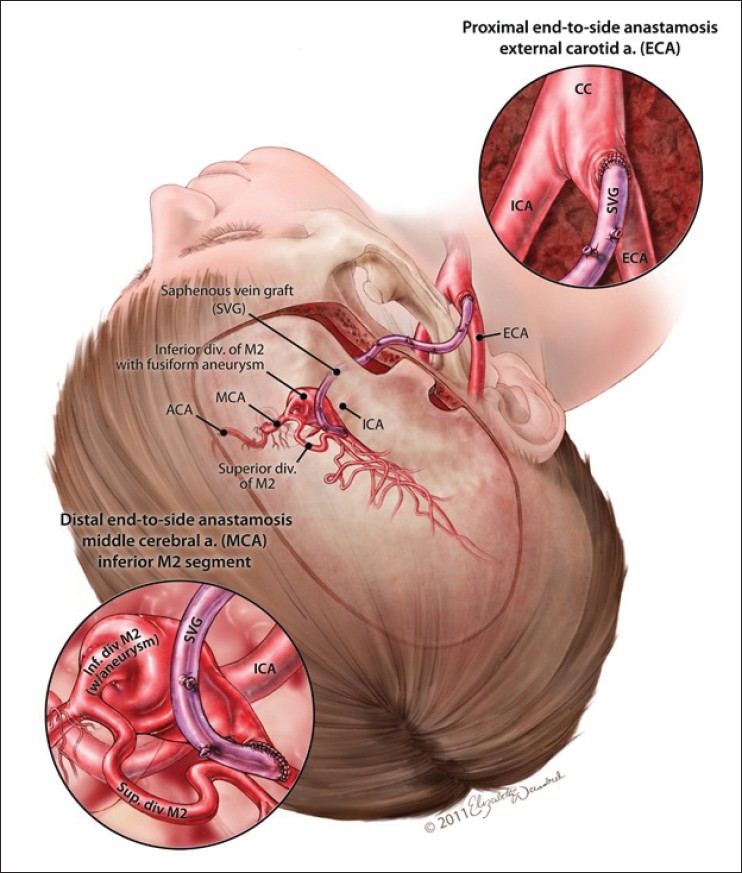 | Figure 3: Artistic rendition of the end to side anastomosis with the ECA
Click here to view |
Postoperative course
The patient was examined and found to be neurologically intact following completion of surgery. Postoperative head CT was stable and demonstrated mild edema in the region of dissection. Graft patency was ensured with biphasic signal on a bedside Doppler ultrasound [Figure 4].
A 5-month postoperative angiogram revealed a patent graft arising from the ipsilateral right ECA with irrigation of the inferior M2 division. There was no evidence of residual filling of the aneurysm. Both clips were visualized on either size of the excluded aneurysmal dilation. There was normal transit time within the right hemisphere.
 Discussion Discussion | |  |
Giant IC aneurysms managed conservatively have 68% mortality at 2 years and 80% mortality at 5 years. [2] The indications for high-flow bypass include giant/fusiform or other unclippable aneurysms and select cranial base tumors that involve the internal carotid or vertebral artery and require resection of parts of these vessels. [3] Various fundamental considerations dictate selection of the appropriate type of bypass for a given situation: Extent of blood flow augmentation required, size of recipient vessel, location of recipient site and anatomic relationship to adjacent perforators or potential intracranial donor vessels, size and availability of donor vessels, and size and availability of graft material. [4] Of the options for high-flow bypass, the most extensive experience has been accrued with radial artery (flow rate, 50 to 150 ml/min) and saphenous vein (flow rate, 100 to 200 ml/min) grafts, with a technique that requires graft vessel harvesting, cervical exposure for proximal anastamosis, and craniotomy for distal anastomosis. [2]
In practice, radial artery and reverse saphenous vein grafts may be used interchangeably in situations where high-flow bypass is required, but various factors may favor the use of one over the other. The radial artery is slightly more difficult and potentially morbid to harvest, and bypass may be complicated by vasospasm. However, radial artery grafts tend to have less size discrepancy at the distal anastomotic site; this may facilitate distal anastomosis, reduce temporary clipping time, and lead to decreased flow turbulence. [5],[6] Furthermore, radial artery grafts are felt to be associated with greater long-term patency than venous grafts which are subject to arterialization and prone to accelerated atherosclerosis. These advantages are mostly theoretical, however, and remain to be rigorously proven; therefore, surgeon preference and training bias largely tend to guide decision-making. [7]
Anastomotic configurations include end-to-end, end-to-side, and rarely, side-to-side as might be utilized in situations of intracranial-intracranial bypass (e.g., A2-A2 bypass). Patient anatomy and surgeon preference help guide the decision of bypass configuration. End-to-end anastomosis may be considered when graft size and recipient vessel size are well matched while end-to-side anastomosis may be preferable when there is significant size mismatch. Some surgeons feel interrupted sutures are superior to a running suture and lead to less constriction at the anastomotic site; a running suture, however, may decrease operative time. Intraoperative angiography is typically used to access graft patency, but small filling defects and sluggish flow may be difficult to appreciate and angiographic quality may be compromised by constraints of positioning for craniotomy. Micro-Doppler probes are an accepted adjunct to assess graft patency and flow rate. The graft may be tunneled in either a preauricular or a retro-auricular fashion as long as kinking and graft compression are assiduously avoided.
With continued technical refinements in microsurgical methods for complex aneurysms, progressively fewer MCA bifurcation aneurysms can truly be classified as "unclippable." [8] In addition to fusiform and giant aneurysms, aneurysms that have perforators or major vessels emanating from the neck or dome may be unsuitable for conventional clip ligation. Various endovascular approaches have increasingly been employed in the treatment of fusiform aneurysms, using balloons and stents or a combination of both methods, and promising results have been published using the recently developed flexible, self-expanding Pipeline stent (ev3, Inc.) to achieve parent vessel reconstruction. [9] Pipeline, however, is not appropriate for bifurcation aneurysms and given the novelty of this technology and lack of long-term follow-up data, there is marginal experience of its use in the pediatric population.
In this case of this child, trapping of the aneurysm located at the proximal inferior M2 division, required a high-flow bypass to a donor site distal to the aneurysm on the inferior M2 division. The superior M2 division was unaffected and the corresponding vascular territory continued to be perfused during temporary clipping. While bypass may be utilized to augment blood flow in adults who undergo EC-IC bypass for ischemia, the specific goal of bypass in this case was to restore blood flow to the inferior M2 division. Because of the risk of developing hand ischemia and surgeon familiarity, a reverse saphenous vein graft was favored over a radial artery graft. The slight size discrepancy of the graft to the inferior M2 recipient site was addressed with an end-to-side anastomosis. Intraoperative Doppler ultrasonography revealed excellent flow rate within the graft following anastomosis and obliteration of the aneurysm was illustrated with intraoperative angiography.
The risk of stroke during high-flow bypass has been variably reported and appears to range anywhere from 6 to 30%. [10],[11],[12] The etiology of ischemia during this procedure may be low flow during temporary clipping; however, embolic stroke from intragraft thrombus formation is also possible. Full heparinization is generally instituted during temporary clipping, as was the case in this patient, to minimize the risk of thromboembolism. A heparin drip (i.e., goal PTT 45-65 s) was continued for 24 hours postoperatively. The patient was also maintained on daily aspirin for antiplatelet effect.
 Conclusion Conclusion | |  |
High-flow bypass for giant/fusiform intracranial pediatric aneurysms can be performed with good outcomes at high-volume neurovascular centers. While a range of controversies continue to circulate regarding optimal surgical techniques, adherence to the basic tenets of blood flow restoration and graft selection are imperative. Although endovascular technology continues to improve, it is likely that high-flow bypass will continue to be required for a relatively small number of patients for many years to come.
 Acknowledgments Acknowledgments | |  |
Disclosures
- The content of this manuscript, in part or in full, has not been published elsewhere in any form.
- This manuscript is a unique submission and is not being considered for publication with any other source in any medium.
- The authors have no other financial or other disclosures to report.
 References References | |  |
| 1. | Sanai N, Auguste KI, Lawton MT. Microsurgical management of pediatric intracranial aneurysms. Childs Nerv Syst 2010;26:1319-27. 
[PUBMED] |
| 2. | Huang J, McGirt MJ, Gailloud P. Intracranial aneurysms in the pediatric population: Case series and literature review. Surg Neurol 2005;63:424-32; discussion 432-3. 
|
| 3. | Newell DW, Skirboll SL. Revascularization and bypass procedures for cerebral aneurysms. Neurosurg Clin N Am 1998;9:697-711. 
[PUBMED] |
| 4. | Sekhar LN, Kalavakonda C. Cerebral revascularization for aneurysms and tumors. Neurosurgery 2002;50:321-31. 
[PUBMED] |
| 5. | Sekhar LN, Duff JM, Kalavakonda C, Olding M. Cerebral revascularization using radial artery grafts for the treatment of complex intracranial aneurysms: Techniques and outcomes for 17 patients. Neurosurgery 2001;49:646-58; discussion 658-9. 
[PUBMED] |
| 6. | Buyukmumcu M, Guney O, Ustun ME, Uysal II, Seker M. Proximal superficial temporal artery to proximal middle cerebral artery bypass using a radial artery graft: An anatomic approach. Neurosurg Rev 2004;27:185-8. 
|
| 7. | Bisson EF, Visioni AJ, Tranmer B, Horgan MA. External carotid artery to middle cerebral artery bypass with the saphenous vein graft. Neurosurgery 2008;62(3 Suppl 1):134-8; discussion 138-9. 
|
| 8. | Lawton MT, Hamilton MG, Morcos JJ, Spetzler RF. Revascularization and aneurysm surgery: Current techniques, indications, and outcome. Neurosurgery 1996;38:83-92; discussion 92-4. 
[PUBMED] |
| 9. | Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol 2011;32:34-40. 
[PUBMED] |
| 10. | Sundt TM Jr, Piepgras DG, Marsh WR, Fode NC. Saphenous vein bypass grafts for giant aneurysms and intracranial occlusive disease. J Neurosurg 1986;65:439-50. 
[PUBMED] |
| 11. | Chibbaro S, Tacconi L. Extracranial-intracranial bypass for the treatment of cavernous sinus aneurysms. J Clin Neurosci 2006;13:1001-5. 
[PUBMED] |
| 12. | Lauder C, Kelly A, Thompson MM, London NJ, Bell PR, Naylor AR. Early and late outcome after carotid artery bypass grafting with saphenous vein. J Vasc Surg 2003;38:1025-30. 
[PUBMED] |
[Figure 1], [Figure 2], [Figure 3], [Figure 4]
|