|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2013 | Volume
: 1
| Issue : 1 | Page : 16-21 |
|
The effect of isoflavones on early bone formation on orthopedically expanded suture in male rats
Faruk Izzet Ucar1, Tancan Uysal2, Abdullah Ekizer3, Mehmet Fatih Sönmez4
1 Private Practice and Erciyes University, Faculty of Dentistry, Kayseri, Turkey
2 Katip Celebi University, Faculty of Dentistry, Izmir, Turkey and Visiting Professor, King Saud University, Riyadh, Saudi Arabia
3 Department of Orthodontics and Erciyes University, Faculty of Dentistry, Kayseri, Turkey
4 Department of Histology and Erciyes University, Faculty of Medicine Kayseri, Turkey
| Date of Web Publication | 18-May-2013 |
Correspondence Address:
Faruk Izzet Ucar
30 Agustos Mah. No: 5/36 38039, Melikgazi, Kayseri
Turkey
 Source of Support: None, Conflict of Interest: None  | Check |
 
Objective: The aim of this experimental study was to evaluate histomorphometrically the effects of isoflavones on bone formation in response to expansion of the inter-premaxillary suture in male rats. Materials and Methods: Twenty male, 50-60 days old Wistar rats were divided into two equal groups (control and experimental). Both groups were subjected to expansion for 5 days, and 50 cN of force was applied to the maxillary incisors with helical spring. In experimental group, 10 μg/g isoflavones (IF) were administrated orally. After expansion period, the springs were removed and replaced with short lengths of rectangular retaining wire for retention period in 10 days. Bone formation in the sutural area was histomorphometrically evaluated including the amount of new bone formation (μm 2 ), number of osteoblasts, number of osteoclasts, and number of vessels. Mann-Whitney U-test was used for statistical evaluation at P < 0.05 level. Results: New bone formation area (P = 0.003; 2.26-fold) and number of osteoclasts (P = 0.001; 1.87-fold) showed statistically significant lower values in experimental group than the control. No significant differences were found in number of osteoblast and number of vessel values between the groups. Conclusion: Isoflavones affects bone formation in the orthopedically expanded inter-premaxillary suture negatively, and main affect of Isoflavones were on osteoclasts in bone metabolism. Keywords: Image analysis, isoflavones, maxillary expansion, rats
How to cite this article:
Ucar FI, Uysal T, Ekizer A, Sönmez MF. The effect of isoflavones on early bone formation on orthopedically expanded suture in male rats. J Orthod Res 2013;1:16-21 |
How to cite this URL:
Ucar FI, Uysal T, Ekizer A, Sönmez MF. The effect of isoflavones on early bone formation on orthopedically expanded suture in male rats. J Orthod Res [serial online] 2013 [cited 2017 Apr 13];1:16-21. Available from: http://www.jorthodr.org/text.asp?2013/1/1/16/112253 |
| Introduction | |  |
Osteoporosis is a skeletal disorder that predisposes to fractures resulting from any particular injury or even minimal trauma. Two major determinants, bone mineral content and bone quality indicates the bone strength. [1]
Estrogen regulates the bone formation and resorption and is also major factor in preserving bone density. [1] It is reasonable to recommend that soy bean or its isoflavones improve bone formation based on at least two evidences: (1) soy isoflavones stimulate osteoblastic activity through activation of estrogen receptors; [2],[3] and (2) soy bean or its isoflavones promote insulin-like growth factor-I (IGF-I) production. [4]
Isoflavones (IF) is found in great amount in soybeans and soy foods and is useful for treatment and prophylaxis of osteoporosis. Soy foods generally contain 1.2-3.3 mg dry weight although exact amount of isoflavones is depending on numerous factors such as range of soy food and geographical location. [5],[6] Studies showed typical isoflavone intakes approximately 30-50 mg/day for Asians [5],[6] and less than 1 mg/day for post-menopausal women in the United States. [7] It has been reported that IF has also various effects, including enhanced osteoblasts activity and inhibiting osteoclasts activity. [8],[9]
In orthodontic treatment, expansion of the mid-palatal suture by rapid maxillary expansion (RME) increases the posterior dentition width rapidly, which is followed by active bone formation in the suture. [10],[11] It is well-known that even following a retention period, the widened maxilla has a strong tendency to return to its previous form. [12] Haas [13] concluded that reorganization of hard tissue in the suture started by the end of active treatment period and ossification of the suture margins is fulfilled in 60-90 days.
Since the relapse is not fully understood, quality and rate of new bone formation in the mid-palatal suture may affect the post-treatment relapse. [14] For that reason, it would be likely favorable to accelerated bone formation in the mid-palatal suture during and after expansion for avoiding relapse of the skeletal base and reduction of the retention period. [11],[14]
In the reviewed literature, there is no published study that evaluated the early effects of isoflavones on bone formation in mid-palatal / inter-premaxillary suture after expansion procedure. The aim of this study was to evaluate histomorphometrically the effects of isoflavones on new bone formation in response to expansion of the inter-premaxillary suture in male rats.
| Materials and Methods | |  |
Animals and diets
Twenty, 50-60 days old Wistar male rats with a mean weight of 248.04 ± 21.76 g were used. All animals were housed in polycarbonate cages, subjected to a 12-hour light-dark cycle at the constant temperature of 23°C and fed a standard pellet diet (Expanded pellets, Stepfield, Witham, Essex, UK) with tap water ad libitum. In experimental group, animals were fed by 10 μg/g IF intraoral gavages method. In present study, isoflavones, dietary supplement (Solgar, USA) were used in concentrated form, which has genistatin and daidzain both. Permission was obtained from the Erciyes University; Ethics Committee of Experimental Animals after the Research Scientific Committee at the same institution had approved the experimental protocol. The experiments were carried out in the Erciyes University.
This study was organized as a parallel group design with one group receiving the experimental protocol and the other receiving the control. The power analysis was performed with G*Power Ver.3.0.10 (Franz Faul, Universitδt Kiel, Germany) software. Based on 1:1 ratio between groups, a sample size of 20 animals would give more than 85% power to detect significant differences with 0.35 effect size and at α = 0.05 significance level. Animals were randomly divided into two groups (control and experimental) of ten rats each.
Appliance placement
The animals were anesthetized with an intramuscular injection of Xylasine (Bayer, Istanbul, Turkey) and Ketamine (Parke-Davis, Istanbul, Turkey) at 0.5 ml/kg and 1 ml/kg body weight, respectively. The expansion appliances were helical springs fabricated from 0.014 inch stainless steel wire inserted in holes drilled close to the gingival margins of both upper incisors [Figure 1]a. The springs were activated to deliver a force of 50 cN and were not reactivated during the 5-day expansion period. After 5 days, the springs were removed and replaced with short lengths of rectangular retaining wire [Figure 1]b. Tooth separation was maintained for 10 days. The distance between the mesial edges of the upper incisors was measured at the beginning of the experiment and at the end of expansion with a digital caliper (MSI-Viking Gage, SC, USA).
Occlusal radiographs (Dexcowin, ADX 4000, Dexcowin Company, Seoul, Korea) were taken from all animals at three stages to check the sutural opening: At baseline, end of expansion, and at the end of the retention period. No measurements were done on these radiographs.
Specimen preparation
After the retention period of 10 days, the rats were sacrificed with an overdose of Ketamine and Xylasine, and their pre-maxillae were dissected out and fixed in 10% formalin. After fixation, the retaining wires were removed, and the pre-maxillae were decalcified with 5% formic acid for four days. Macroscopic evaluation of the specimens was performed before histological evaluation. After decalcification, the pre-maxillae were cut into blocks with one cut passing through the incisor crowns at the alveolar crest and perpendicular to the sagittal plane, the second cut 4 mm apical to the first cut. The sections were rinsed, trimmed, and embedded in paraffin. The paraffin blocks were sectioned serially at 5 μm intervals.
Histomorphometric analysis
The histological sections were stained with hematoxylin and eosin. The histomorphometric measurements were performed 200 μm beneath the oral surface of the osseous palate, because bone formation in the surface layer was sometimes irregular and unsuitable for quantitative measurement.
Stained specimens were investigated by a light microscope (Nikon Eclipse E400, Nikon, Tokyo, Japan). For each specimen, the same area was photographed after staining by using a photograph attachment (Nikon Coolpix 5000; Nikon, Tokyo, Japan). Photographs transferred to a PC environment and calibrated with a Nikon Micrometer Slide (Stage Micrometer Type A, MBM11100, Japan). All photographs were evaluated with an image analysis program (Clemex Vision Lite Image Analysis 3.5; Clemex Technologies, Longueuil, Canada) [Figure 2]. An area of 1.5 mm 2 in the center of the expanded suture was designated for the experimental group. A similarly sized area in the center of the expanded region was assessed for the control group. The amounts of osteoblasts, osteoclasts, and vessels in the newly formed bone region were marked in the 1.5 mm 2 area. Damaged cells were not evaluated. The marked cells were counted automatically with the image analysis program. The amount of new bone formation (μm 2 ) was also measured [Figure 3].  | Figure 2: Histomorphometric measurements of newly formed bone area (μm 2)
Click here to view |
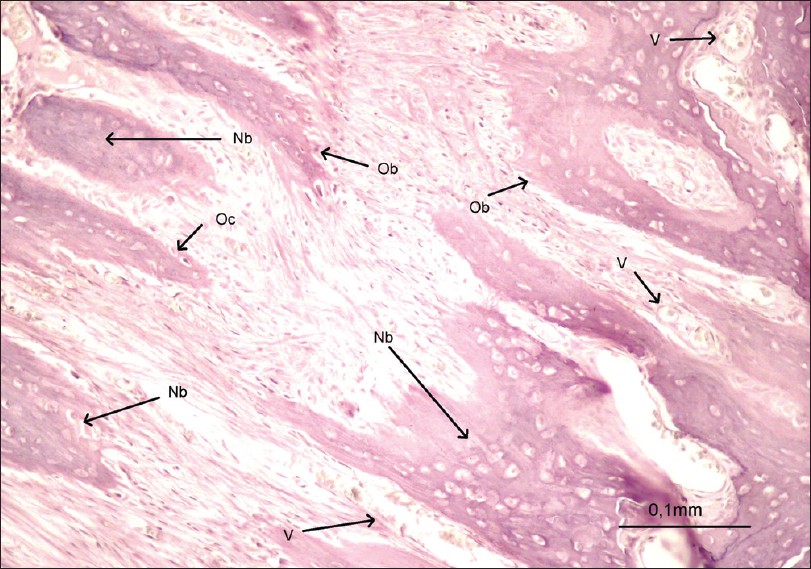 | Figure 3: Photomicrograph of a section in the expansion area (bar = 0,1 mm) Nb: Newbone, Ob: Osteoblast, Oc: Osteoclast, V: Vessel
Click here to view |
Measurements were performed by two assessors who were blinded to the identity of the sections. The final results are averages of these separate evaluations.
Statistical analysis
All data were analyzed with the statistical package for social sciences, 13.0 (SPSS for Windows; SPSS Inc, Chicago, IL, USA). The normality test of Shapiro-Wilks and Levene's variance homogeneity test were applied to the data. The data were not normally distributed, and there was no homogeneity of variance between the groups. Thus, the statistical evaluation was performed using non-parametric tests. Descriptive statistics were given as quartiles (25 th , 50 th -median- and 75 th ). The group differences were studied by the Mann-Whitney U-test. After performing Bonferroni correction, a P value of 0.025 was considered to be significant.
| Results | |  |
All animals survived to the end of the study. Deep mucosal infection, dehiscence or other adverse effects were not observed in any of the animals. The expansion appliances were well-tolerated, and the animals gained weight. As no statistically significant changes in body weight were found during the expansion and retention periods, there was no reason to weight-correct the data.
The histological sections confirmed that the inter-premaxillary sutures were expanded in all groups and there was no statistically significant difference (P = 0.876) in the amount of expansion in the groups [Table 1].  | Table 1: Results and comparisons of biometric analysis for determinati on of the amount of expansion (ƒÝ)
Click here to view |
Statistical analysis showed significant differences between two groups for new bone formation area and number of osteoclasts. New bone formation area (P = 0.003; 2.26-fold), number of osteoclasts (P = 0.001; 1.87-fold) showed lower values in experimental group than the control, and these were found statistically significant [Table 2]. Between the number of osteoblasts and number of vessels were not found statistically significant. For all investigated histomorphometric parameters, IF group showed negative results than the control related to the new bone formation and revealed that bone architecture in the treatment group was getting worse. 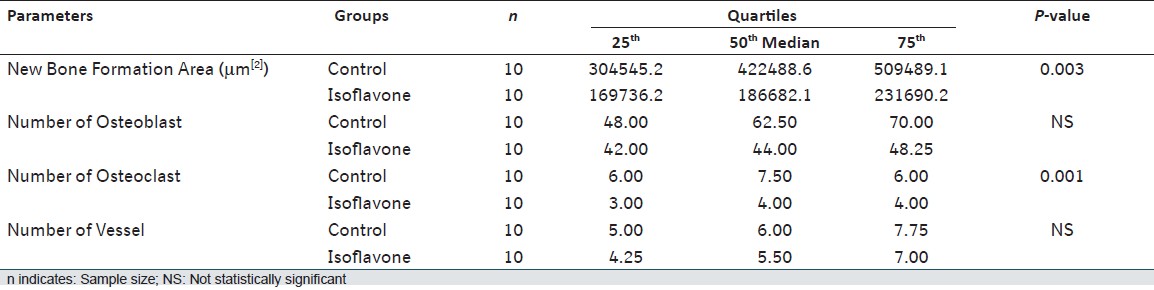 | Table 2: Descriptive values and Mann-Whitney U test comparisons of histomorphometric measurements
Click here to view |
Thus, according to formative changes in new bone formation and number of osteoclasts parameters in IF group, the null hypothesis of this study was rejected.
| Discussion | |  |
The force effects on the rate of bone mineralization can be undertaken by experimental studies on animals. A multifactorial adaptive response takes place in the mid-palatal suture during mid-palatal expansion. Mechanical expansion disrupts the orderly sutural structure and induces a chain of events that restore the suture to its original architecture. [15] Many therapeutic alternatives are being studied to promote cell bio-stimulation, improving the regenerative capacity. [14],[15],[16] Uysal et al.[10] estimated the stimulatory effects of dietary boron in rabbits and locally administered ED-71 [11] in rats on bone formation in response to expansion of the inter-premaxillary suture and found that these mediators could stimulate bone regeneration during expansion and retention phases.
Determining the mechanisms concerned with explanation of the potential benefits of a diet high in plant-based foods continues a main challenge to nutritionists and scientists. IF represent just one of many important bioactive non-nutrients found in many plants commonly consumed in the human diet. IF has main compounds such as genistatin, daidzain, equol, and ipriflavone. In our study, we used genistatin and daidzain together.
Hence, IF is considered an alternative for the treatment of clinical conditions that require tissue regeneration. To our knowledge, this study is the first to report IF effects of active bone formation. Our study showed that application of IF decreased bone healing in the expanded inter-premaxillary suture in the rat histomorphometrically. Reduced new bone formation elements were deposited in the expanded suture following IF application than the control group. This result showed that IF reduced the bone metabolism.
Estrogen plays a major role in preserving bone density by regulating the bone formation and resorption. The chemical structure of IF is likely to our own estrogen. Because of this similarity in structure, they can interfere with the action of our own estrogen. Depending on the type of estrogen-receptor on the cells, IF may decrease or activate the estrogen. IF can compete with estrogen for the same receptor sites, thus decreasing the health risks of excess estrogen. They can also enhance the estrogen activity. [1] Additionally, in line with findings of numerous studies, estrogen suppressed the ovariectomy-induced rise in concentrations of biochemical markers of bone turnover, alkaline phosphatase, and tartrate-resistant acid phosphatase. [17] In our study, we used male rats to eliminate estrogen effects on bone metabolism.
Ovariectomized rats are typically used as an animal model for post-menopausal bone loss. [18] In fact, the attribute of skeletal physiology in the rat model shares similarities with those of early post-menopausal women in many respects, including an increased rate of bone turnover with resorption greater than formation, superior loss of cancellous than cortical bone, and similar skeletal response to plant therapy such as soy IF. [19],[20]
The effects of IF on bone, acting as an inducer of IF, have been studied, [20],[21] but it was considered important to investigate the relationship between dietary composition and maxilla, as there is no known evidence regarding the effect of IF on the structure of the maxilla. It has been shown that IF is highly effective for bone production in long bones, such as the femur and hind limb. [22] However, effects of IF on active bone formation were not examined, thus our investigation was considered to IF effect on the new bone formation on maxilla.
Recently, the bone-sparing effects of soybean IF, either administered orally [4],[19],[23] or injected subcutaneously, [24] have been widely examined in a preventive approach in the most commonly used the ovariectomized rat. [18] Dose-dependent soybean IF is important for the preventive effects on bone metabolism. [25] In our experimental conditions, soy IF were orally given at equivalent amount, i.e., 10 μg/g body weights per day, a dose which has previously been shown to be efficient. [20]
Isoflavones, supplemental dietary, has preventive effects on bone metabolism. In our study, premaksiller expansion requiring active new bone formation bone repair has been the main issue of intensive research. Approaches in clinical use aim to regain function, using materials that replace the damaged tissue rather than regenerating it. Currently, the approach of research regarding bone tissue engineering is to induce regeneration rather than just functional repair. Thus, tissue engineering can now be simply defined as the 'science of persuading the body to heal by its intrinsic repair mechanisms.' [26] The complexity of skeletal tissues has been hindering the development of an effective regeneration system. Nevertheless, huge steps are being taken regarding the use of progenitor/stem cells, adequate scaffold materials, and growth factors/bioactive agents. The combination in a single system of properties including structural support, cell support, and controlled release is the way to go, and materials in the particulate form have all the potential needed for achieving such a goal. [27]
| Conclusion | |  |
- Isoflavones affects bone formation in the orthopedically expanded inter-premaxillary suture negatively, during expansion and early phase of retention periods.
- These findings suggest that the main effect of isoflavones was on osteoclasts in bone metabolism.
Clinical relevance
This study confirmed that isoflavones could be nudged into osteogenic activity by means of reducing bone metabolism. It was demonstrated that of oral administration of isoflavones during expansion inhibited bone formation. This principle should not be applied during distraction osteogenesis or for the treatment of patients with long bone fractures fixed by external devices or treating fractures with delayed union and non-union.
| References | |  |
| 1. | Bawa S. The significance of soy protein and soy bioactive compounds in the prophylaxis and treatment of osteoporosis. J Osteop 2010;8:1-8. 
|
| 2. | Ma DF, Qin LQ, Wang PY, Katoh R. Soy isoflavone intake inhibits bone resorption and stimulates bone formation in menopausal women: meta-analysis of randomized controlled trials. Eur J Clin Nutr 2008;62:155-61. 
|
| 3. | Choi EM, Suh KS, Kim YS, Choue RW, Koo SJ. Soybean ethanol extract increases the function of osteoblastic MC3T3-E1 cells. Phytochemistry 2001;56:733-9. 
|
| 4. | Arjmandi BH, Getlinger MJ, Goyal NV, Alekel L, Hasler CM, Juma S,et al. The role of soy protein with normal or reduced isoflavone content in reversing bone loss induced by ovarian hormone deficiency in rats. Am J Clin Nutr 1998;68:1358-63. 
|
| 5. | Wang H, Murphy PA. Isoflavone content in commercial soybean foods. J Agric Food Chem 1994;42:1666-73. 
|
| 6. | Wang H, Murphy PA. Isoflavone composition of American and Japanese soybeans in Iowa: effects of variety, crop year, and location. J Agric Food Chem 1994;42:1674-7. 
|
| 7. | Cornwell T, Cohick W, Raskin I. Dietary phytoestrogens and health. Phytochemistry 2004;65:995-1016. 
|
| 8. | Tsuda M, Kitazaki T, Ito T, Fujita T. The effect of ipriflavone (TC-80) on bone resoiption in tissue culture. J Bone Miner Res 1986;1:207-11. 
|
| 9. | Ushiroyama T, Okamura S, Ikeda A, Ueki M. Efficacy of ipriflavone and 1 alpha vitamin D therapy for the cession of vertebral bone loss. Int J Gynaecol Obstet 1995;48:283-8. 
|
| 10. | Uysal T, Ustdal A, Sonmez MF, Ozturk F. Stimulation of bone formation by dietary boron in an orthopedically expanded suture in rabbits. Angle Orthod 2009;79:984-90. 
|
| 11. | Uysal T, Amasyali M, Enhos S, Sonmez MF, Sagdic D. Effect of ED-71, a new active vitamin D analog, on bone formation in an orthopedically expanded suture in rats. A histomorphometric study. Eur J Dent 2009;3:165-72. 
|
| 12. | Krebs AA. Midpalatal suture expansion studied by the implant method over a seven-year period. Trans Eur Orthod Soc 1964;40:131-42. 
|
| 13. | Haas AJ. The treatment of maxillary deficiency by opening the midpalatal suture. Angle Orthod 1965;35:200-17. 
|
| 14. | Saito S, Shimizu N. Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofacial Orthop 1997;111:525-32. 
|
| 15. | Chang HN, Garetto LP, Potter RH, Katona TR, Lee CH, Roberts WE. Angiogenesis and osteogenesis in an orthopedically expanded suture. Am J Orthod Dentofacial Orthop 1997;111:382-90. 
|
| 16. | Kawasaki K, Shimizu N. Effects of low-energy laser irradiation on bone remodeling during experimental tooth movement in rats. Lasers Surg Med 2000;26:282-91. 
|
| 17. | Raisz LG. Local and systemic factors in the pathogenesis of osteoporosis. N Engl J Med 1988;318:818-28. 
|
| 18. | Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner 1991;15:175-91. 
|
| 19. | Arjmandi BH, Alekel L, Hollis BW, Amin D, Stacewicz-Sapuntzakis M, Guo P, Kukreja SC. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J Nutr 1996;126:161-7. 
|
| 20. | Picherit C, Coxam V, Bennetau-Pelissero C, Kati-Coulibaly S, Davicco MJ, Lebecque P, et al. Daidzein is more efficient than genistein in preventing ovariectomy-induced bone loss in rats. J Nutr 2000;130:1675-81. 
|
| 21. | Agnusdei D, Gennari C, Bufalino L.Prevention of early postmenopausal bone loss using low doses of conjugated estrogens and the non-hormonal, bone-active drug ipriflavone. OsteoporosInt 1995;5:462-6. 
|
| 22. | Yamazaki I. Shino A, Shimizu Y, Tsukuda R. Shirakawa Y, Kinoshita M.Effect of ipriflavone on glucocorticoid-induced osteoporosis in rats. Life Sci 1986;38:951-8. 
|
| 23. | Anderson JJ, Ambrose WW, Garner SC. Biphasic effects of genistein on bone tissue in the ovariectomized lactating rat model. Proc Soc Exp Biol Med 1988;217:345-50. 
|
| 24. | Fanti P, Monier-Faugere MC, Geng Z, Schmidt J, Morris PE, Cohen D, Malluche HH. The phytoestrogen genistein reduces bone loss in short-term ovariectomized rats. OsteoporosInt 1998;8:274-81. 
|
| 25. | Picherit C, Bennetau-Pelissero C, Chanteranne B, Lebecque P, Davicco MJ, Barlet JP, et al. Soybean ýsoflavones dose-dependently reduce bone turnover but do not reverse established osteopenia in adult ovariectomized rats. J Nutr 2001;131:723-8. 
|
| 26. | Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res 2001;55:141-50. 
|
| 27. | Silva GA, Coutinho OP, Ducheyne P, Reis RL. Materials in particulate form for tissue engineering. 2. Applications in bone. J Tissue Eng Regen Med 2007;1:97-109. 
|
[Figure 1], [Figure 2], [Figure 3]
[Table 1], [Table 2]
|