NEURONAL PDZ DOMAINS: A Promising New Molecular Target for Inhaled Anesthetics?
- Yuan-Xiang Tao, MD, PhD and
- Roger A. Johns, MD
Abstract
Despite widespread use of volatile general anesthetics in millions of patients each year, the mechanisms by which they exert multiple effects on the behavior of central neurons are poorly understood. PDZ [postsynaptic density 95 (PSD-95), discs large (Dlg), and zonula occludens-1 (ZO-1)] domains are ubiquitous protein interaction modules that participate in neuronal signaling. Recent studies have indicated that clinically relevant concentrations of inhaled anesthetics dose-dependently and specifically inhibit the PDZ domain–mediated protein interactions among multiprotein signaling complexes. These inhibitory effects are immediate, potent, reversible, and occur at a hydrophobic peptidebinding groove on the surface of the PDZ domain. Thus, the PDZ domain might be a new molecular target for inhalational anesthetics.
Introduction
The mechanisms of general anesthetic action at the molecular level remain poorly understood, despite their widespread use for more than 150 years. Early hypotheses that lipid-soluble anesthetics act via nonspecific interactions with membrane bilayers have largely given way to the recent suggestion that neuronal membrane-associated proteins, particularly ion channels and receptors, are specifically targeted by anesthetics (1). A working hypothesis is that inhaled anesthetics enhance inhibitory postsynaptic channel activity [i.e., γ-amino butyric acid subtype A (GABAA) and glycine receptors] and inhibit excitatory synaptic channel activity (i.e., nicotinic acetylcholine, serotonin, and glutamate receptors) (1).
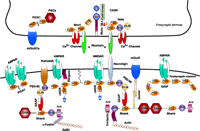
In the central nervous system, synapses, highly specialized sites of contact between neurons, are organized to facilitate the transmission of signals from the presynaptic terminal to the postsynaptic membrane and to activate subsequent signal transduction cascades that result in appropriate cellular events. Efficient and precise organization of synaptic proteins such as receptors, ion channels, and signaling molecules at both presynaptic and postsynaptic membranes is critical for proper signal transmission (2, 3). Over the last several years, studies have revealed a much more complicated picture of ion channel–mediated synaptic transmission than previously anticipated. For efficient synaptic transmission, the receptors, ion channels, and their downstream signaling proteins are clustered or anchored at neuronal synapses via a complex network of protein–protein interactions that govern many critical physiological and pathological processes (4). The PDZ [postsynaptic density 95 (PSD-95), discs large (Dlg), and zonula occludens-1 (ZO-1)] domain is one of the most common protein–protein recognition modules in the nervous system (Figure 1⇓) (5), and new research suggests that synaptic PDZ domains might be new molecular targets for inhaled anesthetics (6, 7)
Neuronal PDZ domain–organized macromolecular signaling complexes at the synapse. The intercellular junction is formed by neurexin and neuroligin. On the presynaptic side, CASK interacts with neurexin through its PDZ domain. CASK also interacts with two PDZ domain–containing proteins, Velis and Mint1, and with N-type Ca2+ channels. The PDZ domains of Mint1 bind to N-type Ca2+ channels, and its N-terminus interacts with Munc 18-1 and CASK. In addition, the PDZ domain of PICK1 (protein interacting with C kinase-1) interacts with the C terminus of metabotropic glutamate receptor subunit 7a (mGluR7a) and the catalytic subunit of protein kinase Cα (PKCα). On the postsynaptic side, the PDZ domains of PSD-95 interact with the PDZ domain of neuronal nitric oxide synthase (nNOS) and with the C-termini of the N-methyl-d-aspartate receptor (NMDAR), neuroligin, and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor (AMPAR)- targeting protein stargazin. The Src homology 3 (SH3) domain of PSD-95 binds to kainate receptors (KainateR). PSD-95 is attached to the postsynaptic membrane via the N-terminal palmitate group (wiggly line). The guanylate kinase (GUK) domain of PSD-95 interacts with guanylate kinase domain-associated protein (GKAP), and the C-terminal tail of GKAP directly binds to the PDZ domain of Shank. Shank also couples the metabotropic glutamate receptors (mGluRs) via the bridge protein Homer. Shank can multimerize via its sterile alpha motif (SAM) domain and is directly linked to the cytoskeleton via two actin-binding proteins: cortactin and α-fodrin. The AMPA receptors also bind to two additional PDZ proteins GRIP/ABP (glutamate receptor-interacting protein/AMPA receptor–binding protein) and PICK1 via PDZ domain-mediated protein interactions. MI, Munc 18-1-interaction domain; CI, CASK interaction domain; Ank, ankyrin repeat.
PDZ Domain Structure And Its Ligand Recognition
The identification of PDZ domains was based on the high incidence of sequence similarity and identity within a segment of 80–100 residues shared by the three proteins, referred to above, that give their name to the protein family (5). They were originally named the Discs-large homology regions (DHRs) or the GLGF repeat (after the highly conserved Gly-Leu-Gly-Phe motif within the domain) (5).
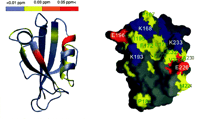
X-ray crystallographic and NMR spectroscopic techniques have helped solve the structures of a number of canonical PDZ domains, both in free and bound forms. The first PDZ domain crystal structure was solved for the third PDZ domain of PSD-95 (8, 9). Since that time, numerous additional PDZ crystal structures have been determined in recent years, including the single PDZ domains of CASK, syntrophin, and neuronal nitric oxide synthase (nNOS); the first PDZ domain of the Na+-H+ exchanger regulatory factor (NHERF); the first PDZ domain of InaD (the PDZ domain–containing scaffolding protein found in the Drosophila phototransduction complex); the second PDZ domain of PSD- 95; and the second PDZ domain of both the human and mouse protein tyrosine phosphatase orthologs PTP1E/PTP-BL (8, 9). The common structure of PDZ domains comprises six β strands (βA- βF) and two α helices (αA and αB), which fold in an overall six-stranded β sandwich (Figure 2⇓). PDZ domains specifically recognize short C-terminal peptide motifs that are often found in the cytoplasmic tails of transmembrane receptors and channels (8, 9). Short C-terminal peptide motifs bind in an extended groove between the second α-helix (αB) and the second β-strand (βB). Amino acid residues at the 0 and −2 positions [0 refers to the last (i.e., C-terminal) residue, which contains a free carboxyl group, and −2 is the residue located two positions up toward the N terminus] in the C-terminal motif confer much specificity in the peptide’s binding to a cognate PDZ domain, although residues at the −1 and −3 positions and those further toward the N terminus also contribute to the binding. Despite similarities in secondary structure and the common preference for C-terminal ligands, PDZ domains display different binding specificity.
Chemical-shift changes of PSD-95 PDZ2 resulting from halothane binding. A. The horizontal bar at the top of the figure represents the coloring scheme. The figure was prepared using the program MOLMOL. The red color represents the largest changes, followed by orange, and then yellow. The gray means no change after adding halothane. The peptide-binding groove of PSD-95 PDZ2 had the most extensive and largest changes, indicating it is also the halothane binding site. B. Surface representation of the target-binding pocket of PSD-95 PDZ2. The hydrophobic residues are shown in yellow, negatively charged residues in red, positively charged residues in blue, and the polar amino acids in gray. The orientation of the PDZ domain is identical to that shown on left. Reprinted by permission of the
Generally, PDZ domains are classified into three types according to their specificity for C-terminal peptide ligands (Table 1⇓). In type I PDZ interactions, such as those of PSD-95, a serine or threonine residue occupies the −2 position of the C-terminal ligand. This type of PDZ domain is specific for -S/T-X-Φ target sequence (X: unspecified amino acid; Φ: hydrophobic amino acid) (10). In contrast, the second type of PDZ domain (such as that of PICK1), specific for -X-Φ-X-Φ sequences, is characterized by hydrophobic residues at both the −2 position of the peptide ligand and the αB1 position of the PDZ domain (10). A third type of PDZ domain, such as that of nNOS, is specific for a different -X-D/E-X-Φ pattern, and prefers negatively charged amino acids at the −2 position (11).
Classification of PDZ Domains Based on the C-terminal Sequence of Their Binding Partners
PDZ Domain-Containing Proteins In Neuronal Signaling
With the sequences of several genomes now available, we know of 440 PDZ domains in 259 different human proteins, 133 PDZ domains in eighty-six Drosophila proteins, and 138 PDZ domains in ninety-six Caenorhabditis elegans proteins (12). These PDZcontaining proteins can be classified into three principal families according to their organization properties (9, 13). Class I PDZcontaining proteins neither contain intrinsic enzyme activities nor have enzyme-like domains. This class of PDZ proteins can be further divided into two subclasses, I-1 and I-2. A striking feature of class I-1 is that these proteins contain only PDZ domains and no other recognizable protein interaction domains. For example, glutamate receptor-interacting protein [(GRIP), an α-amino-3- hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptorinteracting protein] possesses seven PDZ domains, and NHERF contains two PDZ domains.
In class I-2 PDZ proteins, PDZ domains frequently appear together with other protein domains, but again, without detectable enzyme activities. Examples include members of the PSD-95 family: PSD-95/SAP90 (14, 15), PSD-93/chapsyn-110 (16, 17), SAP-97/hdlg (18), and SAP102 (19). These proteins consist of three tandem PDZ domains at the N-terminal side, a Src homology region 3 (SH3) domain in the middle, and a guanylate kinase (GUK)-like domain toward the C terminus. The GUK domain lacks catalytic activity, and is a protein-binding module.
In contrast, the class II PDZ proteins contain one or at most two catalytic domains. Various types of enzymatic domains can coexist with PDZ domains. For example, PDZ domains can occur in both protein kinases and phosphatases. nNOS contains a PDZ domain toward its N terminus, and the function of this PDZ domain is to physically target the enzyme to the N-methyl- d-aspartate (NMDA) receptor by binding to the PDZ domain of PSD-95 or PSD-93 at synapses (20).
The third class is composed of proteins harboring unconventional PDZ domains. Amino acid sequence analyses have identified a distinct family of “PDZ-like” domain–containing proteins in plants, bacteria, as well as metazoans (13). Of interest to this review are PDZ proteins located at the synapses of central neurons in mammals.
PDZ domains are crucial organizers of protein complexes and are important in transport and targeting of different proteins to the sites of neuronal signaling at synapses (Figure 1⇑) (4, 5). At the presynaptic nerve terminal, a set of proteins, including PDZcontaining proteins such as PICK1, CASK, Mint proteins, Velis, and β-catenin, surround the active zone where neurotransmitters are released (Figure 1⇑) (21–27). These PDZ domain-containing proteins are involved in synaptic vesicle localization, transportation, and exocytosis. For example, Mint proteins specifically expressed in brain are composed of an N-terminal sequence that simultaneously binds Munc 18-1 and CASK, a middle phosphotyrosine- binding domain (PTB), and two C-terminal PDZ domains that directly bind to neurexins (Figure 1⇑) (24–27). CASK is a PDZcontaining protein that, in turn, binds to neurexins and to Mint1 and Velis (26, 27). Both Munc 18-1 and neurexins are essential for synaptic vesicle exocytosis. Studies demonstrate that Mint proteins cluster the Munc 18-1 and neurexins to the sites of exocytosis and are involved in presynaptic release of neurotransmitters (24–27). This view is supported by a study showing impaired GABAergic synaptic transmission in Mint1 knockout mice (28). Another presynaptic PDZ domain protein, PICK1, interacts with PKCα and the metabotropic glutamate receptor 7a (mGluR7a) in presynaptic terminals. This interaction is required for inhibiting mGluR7amediated synaptic transmission (21, 22). In the postsynaptic nerve terminal, the postsynaptic density beneath the membrane contains an impressive array of PDZ-containing proteins (6, 8, 9). By way of PDZ domain-mediated interactions, these proteins not only cluster or scaffold neurotransmitter receptors at the postsynaptic membrane, but also assemble a set of specific intracellular proteins around the receptors and mediate neuronal signal transmission (Figure 1⇑) (6, 8, 9). One of the best-understood PDZ domain–containing proteins in synaptic signal transduction is PSD-95, a modular protein that is abundantly present in the postsynaptic density (5). As discussed above, PSD-95 is one member of a family of related proteins. The first and second PDZ domains of PSD-95, PSD-93, and SAP102 interact with the S/T-X-V-COOH motif of the C-termini of NR2A and NR2B subunits of NMDA receptors (5, 8, 9). The second PDZ domain found in PSD-95 and in PSD-93 also can form heterodimeric PDZ–PDZ interactions with the PDZ domain of nNOS (8, 9). The coupling of nNOS to the NMDA receptor by PSD-95’s second PDZ domain facilitates NMDA-mediated activation of nNOS, which is critical to neuronal plasticity, learning, memory, and behavior (29, 30). Mice harboring mutations in the PSD-95 gene display altered long-term potentiation, impaired learning, and disrupted neuropathic sensitization of behavioral reflexes (31, 32). Reduced expression of PSD-95 (i.e., genetic “knockdown”) blocks NMDA receptor-dependent nitric oxide production and subsequent excitotoxicity (33). Perturbing NMDA receptor–PSD-95 protein interactions with fusion proteins that encode either the C-terminal motif of NR2B or the first two PDZ domains of PSD-95 not only protects cultured neurons from excitotoxicity and reduces focal ischemic brain damage, but also attenuates NMDA-induced cGMP production (34). Targeted disruption of the PSD-93 gene alters the surface expression of NR2A and NR2B, impairs NMDA receptor synaptic function, and blunts central pain signaling transmission (35). PDZ domain–mediated protein interaction with the NMDA receptor complex at synapses is critical not only for synaptic NMDA receptor localization and function, but also for assembly of NO signaling around the NMDA receptor during physiological and pathological processes. In addition to binding to NMDA receptors and to nNOS, PSD-95 binds to other receptors and channels, and intracellular proteins, such as β1-adrenergic receptors (36), both voltage-gated and inwardly rectifying K+ channels (37, 38), guanylyl cyclase (39), and SynGAP (40). It should be noted that the third PDZ domain of PSD-95 binds to neuroligins, which bind to pre-synaptic neurexins, and are thought to perform important functions in shaping synapses (41). The PDZ domains of other proteins also mediate protein–protein interactions in the postsynaptic signaling complexes, such as between AMPA receptor subunit GluR2 and GRIP or PICK1 (42) and between Homer and mGluR (43). Shank, a PDZ-containing protein, links the NMDA receptor complex with the mGluR signaling pathway through Shank’s interaction with Homer and GUK-associated protein (GKAP), which binds to the GUK domain of PSD-95 (44). Thus, PDZ domain–mediated protein-protein interactions have been identified in many diverse synaptic signal transduction pathways at both pre- and post-synaptic sites of the central nervous system.
Interaction Of Inhaled Anesthetics With Neuronal PDZ Domains
Anesthetics exert multiple effects on the central nervous system. The current hypothesis is that anesthetics primarily modulate multiple membrane-associated proteins involved in synaptic transmission (1). Thus, inhibitory GABAA and glycine receptors, and excitatory NMDA, nicotinic acetylcholine, and serotonin receptors are possible physiological targets underlying general anesthesia (1). New research has revealed that inhaled anesthetics might affect neuronal signaling transmission by binding to PDZ domains. Clinically relevant concentrations of inhaled anesthetics, including halothane, isoflurane, and sevoflurane, dose-dependently and specifically inhibit PDZ domain-mediated protein interaction between PSD-95 or PSD-93 and the NMDA receptor, nNOS, or the Kv1.4 channel, as well as between PICK1 and AMPA receptor subunit GluR2 without affecting non-PDZ domain–mediated protein interaction between the GUK domain of PSD-95 and SH3 domain of SAP102 (6). Halothane also disrupts the physiological complex of PSD-95 and the NMDA receptor in brain tissue (6)––these inhibitory or disruptive effects are immediate, potent, and reversible. Moreover, NMR data have revealed that halothane is likely to bind to a hydrophobic pocket formed by amino acid residues L170, I172, I174, I195, V229, and L232 (Figure 2⇑) (6). The two positively charged residues located in the vicinity of the hydrophobic pocket also play a positive role in stabilizing the electron-rich halothane molecule (1). The halothane-binding site on the second PDZ domain of PSD-95 directly overlaps with the binding pocket for nNOS and for the NMDA NR2 subunit (6). Inhaled anesthetics might directly compete with these PDZ domain-binding motifs to disrupt synaptic signal transmission and to contribute to the anesthetic state. The relevance of the PDZ domains in the anesthetic state is also suggested by in vivo studies showing that the deficiency of PDZ domain-containing proteins reduces the minimum alveolar anesthetic concentration (MAC) for anesthesia to occur (7) and produces antinociception in numerous pain models (32, 35, 45–49). The results described above suggest that neuronal PDZ domains might be new molecular targets for inhaled anesthetics; however, the detailed biophysical interaction between the inhaled anesthetics and the PDZ domains is still unclear. Further studies using point mutations of the PDZ domains will provide more information about the structural requirements of the PDZ domains for specific anesthetic binding. In addition, PDZ domains display different binding affinity and specificity as discussed above (Table 1⇑). It will be interesting to investigate the effects of the inhaled anesthetics on other PDZ domain–mediated protein interactions at synapses, particularly on the presynaptic side. Studies such as these will elucidate whether inhaled anesthetics affect multiple pre- and post-synaptic neuronal signaling events via PDZ domain–mediated protein interactions in the central nervous system.
Conclusion
Although the physiological significance of the disruption of these PDZ domain interactions by inhaled anesthetics remains to be explored, new research indicates that inhaled anesthetics interact with neuronal PDZ domains in the nervous system. This knowledge may lead to a new concept for anesthetic action in which the general anesthetic state is achieved not only by altering synaptic receptor activities but also by affecting PDZ domain–mediated protein–protein interactions. This knowledge may also contribute to the design of small molecular weight compounds that are clinically useful to decrease the need for inhaled anesthetics and to reduce postoperative pain.
Acknowledgments
This work was supported by the Johns Hopkins University Blaustein Pain Research Fund, and the National Institutes of Health Grants R01 GM 49111 and NS 44219.
- © American Society for Pharmacology and Experimental Theraputics 2004
References

Roger A. Johns, MD, is a Professor in the Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA. His research interests include the molecular mechanisms of anesthesia and analgesia, and interactions between endothelium and vascular smooth muscle in vascular remodeling.

Yuan-Xiang Tao, MD, PhD, is an Assistant Professor in the Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA. He is leading a research team to investigate the roles of neuronal PDZ domain proteins in the molecular mechanisms of anesthesia and analgesia. His research interests include molecular and synaptic mechanisms of anesthesia and pathological pain. Please address correspondence to Y-XT. E-mail ytau{at}jhmi.edu; fax 410-614-7711.