|
 
 |
| ORIGINAL ARTICLE |
|
| Year : 2014 | Volume
: 9
| Issue : 1 | Page : 31-33 |
|
Response of the pulmonary system to exercise in proliferative phase of menstrual cycle in obese and non obese women
A Pakkala1, CP Ganashree2, T Raghavendra3
1 Department of Physiology, PES Institute of Medical Sciences and Research, Kuppam, Andhra Pradesh, India
2 Department of Physiology, Basaveshwara Medical College, Chitradurga, Karnataka, India
3 Department of Anesthesiology, Basaveshwara Medical College, Chitradurga, Karnataka, India
| Date of Web Publication | 15-May-2014 |
Correspondence Address:
A Pakkala
Department of Physiology, PES Institute of Medical Sciences & Research, Kuppam, Andhra Pradesh, 40, SM Road 1st Cross, T. Dasarahalli, Bangalore - 560 057, Karnataka
India
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/9783-1230.132556

Background: The role of estrogen on pulmonary function test (PFT) was well known in the normal course of the menstrual cycle. Significant increase in both progesterone (37%) and estradiol (13.5%), whereas no change in plasma follicle stimulating hormone (FSH) & leutinizing hormone [LH] was observed in exercising women in previous studies. Therefore, this study was intended to see the limitations of the pulmonary system in adaptability to exercise in proliferative phase of menstrual cycle in obese and non obese women. Materials and Methods: Healthy young adult females between 19-25 years in proliferative phase of menstrual cycle leading a sedentary life style were considered in the study group.10 subjects in each group were studied based on the body mass index (BMI), were made to undergo treadmill exercise testing and computerized spirometry to assess dynamic lung functions. Results: It was observed that exercise per se does not cause a statistically significant change in dynamic lung function parameters maximum mid expiratory flow [MMEF], peak expiratory flow rate (PEFR), mid expiratory flow (MEF) 25% to 75% in either of the groups. Conclusion: This finding supports the hypothesis that the respiratory system is not normally the most limiting factor in the delivery of oxygen even under the predominant influence of estrogen in proliferative phase which is further accentuated by exercise and obesity, at least borderline does not have much influence on respiratory system adaptability. Keywords: Adaptability, Estrogen in exercise, Proliferative phase, PFT
How to cite this article:
Pakkala A, Ganashree C P, Raghavendra T. Response of the pulmonary system to exercise in proliferative phase of menstrual cycle in obese and non obese women. J Med Investig Pract 2014;9:31-3 |
How to cite this URL:
Pakkala A, Ganashree C P, Raghavendra T. Response of the pulmonary system to exercise in proliferative phase of menstrual cycle in obese and non obese women. J Med Investig Pract [serial online] 2014 [cited 2018 Aug 24];9:31-3. Available from: http://www.jomip.org/text.asp?2014/9/1/31/132556 |
| Introduction | |  |
The role of hormones on the healthy pulmonary system in delivering oxygen to meet the demands of various degrees of exercise has been a matter of differences in opinion. There are conflicting reports that the respiratory system is not normally the most limiting factor in the delivery of oxygen to the muscles during maximal muscle aerobic metabolism whereas others do not subscribe to this. [1] Within this context it is appropriate to study the effect of proliferative phase of menstrual cycle on ventilatory functions after exercise.
Mechanical constraints on exercise hyperpnoea have been studied as a factor limiting performance in endurance athletes. [ ] Others have considered the absence of structural adaptability to physical training as one of the "weaknesses" inherent in the healthy pulmonary system response to exercise. [≥ ]
Ventilatory functions are an important part of functional diagnostics, [4] aiding selection and optimization of training and early diagnosis of sports pathology. Assessment of exercise response of dynamic lung functions in the healthy pulmonary system in the trained and the untrained has a role in clearing gaps in the above areas especially a special group like peri-menopausal women.
| Materials and Methods | |  |
The present study was conducted as a part of cardio-pulmonary efficiency studies on two groups of non-obese (n=10) and obese (n=10) comparable in age and sex shown in [Table 1].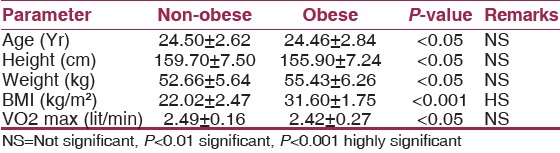 | Table 1: Comparison of anthropometric data & VO2 max of non-obese and obese with statistical analysis
Click here to view |
Informed consent was obtained and clinical examination to rule out any underlying disease was done. Healthy young adult females between 19-25 years who lead a sedentary lifestyle were considered in the study group. Obesity was determined by body mass index [BMI]. They did not have any such regular exercise program. Smoking, clinical evidence of anemia, obesity, involvement of cardio-respiratory system was considered as exclusion criteria. Menstrual history was ascertained to confirm proliferative phase of menstrual cycle.
Detailed procedure of exercise treadmill test and computerized spirometry was explained to the subjects.
Dynamic lung functions were measured in both groups before exercise was evaluated following standard procedure of spirometry using computerized spirometer Spl-95. All subjects were made to undergo maximal exercise testing to VO2 max levels on a motorized treadmill.
After exercise, the assessment of dynamic lung functions was repeated. All these set of recordings were done on both the non-athlete as well as the athlete groups.
Statistical analysis was done using paired students t-test for comparing parameters within the group before and after exercise testing and unpaired t-test for comparing the two groups of subjects.
A P < 0.01 was considered as significant.
| Discussion | |  |
Considerable information can be obtained by studying the exercise response of dynamic lung functions in untrained and trained subjects.
Intra group comparison is helpful in noting the exercise response and inter-group comparison in evaluating adaptations of the respiratory system to training.
On comparing the anthropometric data of the two study groups it is clear that the age and sex matched subjects have no statistically significant difference in height. BMI determined the obese status.
Maximum oxygen consumption [VO2 max] values were not significantly different in the two groups. This observation is expected in view of the similar sedentary lifestyle of the two groups and adaptability of both the pulmonary system and the cardio vascular system. VO2 max is an objective index of the functional capacity of the body's ability to generate power.
Forced vital capacity (FVC) is the volume expired with the greatest force and speed from total lung capacity [TLC] and forced expiratory volume [FEV1] that expired in the 1 st second during the same maneuver. The FEV1 was initially used as an indirect method of estimating its predecessor as the principal pulmonary function test, the maximal breathing capacity. [5]
On comparing the response of exercise within the two study groups and in between them, there is no statistically significant difference in forced vital capacity [FVC] and FEV1 under any condition.
A normal FEV1/FVC ratio is observed always.
Another way of looking at forced expiration is to measure both expiratory flow and the volume expired. The maximum flow obtained can be measured from a flow - volume curve is the peak expiratory flow rate (PEFR). The peak flow occurs at high lung volumes and is effort dependent. Flow at lower lung volumes is effort independent. Flow at lower lung volumes depends on the elastic recoil pressure of the lungs and the resistance of the airways upstream or distal to the point at which dynamic compression occurs. Measurements of flow at low lung volumes, mid expiratory flow [MEF 25% to 75%] are often used as indices of peripheral or small airways resistance. [5],[6]
On examining [Table 2] and [Table 3] it is clear that exercise per se does not cause a statistically significant change in dynamic lung function parameters MMEF, PEFR, MEF 25% to 75% in either of the groups. This finding supports the hypothesis that the respiratory system is not normally the most limiting factor in the delivery of oxygen. | Table 2: Comparison of Dynamic Lung Functions of Non- obese before exercise testing (BE) and after exercise testing (AE) with statistical analysis
Click here to view |
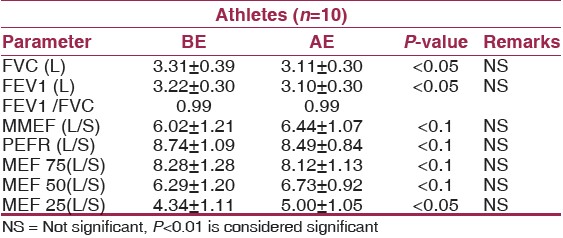 | Table 3: Comparison of Dynamic Lung functions of Obese before exercise testing (BE) and after exercise testing (AE) with statistical analysis
Click here to view |
Thirty minutes of exercise at 74% of VO2 was found to cause a significant increase in both progesterone (37%) and estradiol (13.5%), whereas no change in plasma follicle stimulating hormone [FSH] and leutinizing hormone [LH] was observed in exercising women, [7] others have confirmed these findings. [8] This finding supports the hypothesis that the respiratory system is not normally the most limiting factor in the delivery of oxygen even under the predominant influence of estrogen in proliferative phase which is further accentuated by exercise and obesity, at least borderline does not have much influence on respiratory system adaptability.
| conclusion | |  |
This finding supports the hypothesis that the respiratory system is not normally the most limiting factor in the delivery of oxygen even under the predominant influence of estrogen in proliferative phase which is further accentuated by exercise and obesity; at least borderline does not have much influence on respiratory system adaptability.
| References | |  |
| 1. | Guyton AC, Hall JE, editors. Text Book of Medical Physiology. 11 th ed. Philadelphia: Saunders: 2006. p. 1061-2. 
|
| 2. | Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hypernea in endurance athletes. J Appl Physiol 1992;73:874-6. 
|
| 3. | Dempsey JA, Johnson BD, Saupe KW. Adaptations and limitations in the pulmonary system during exercise. Chest 1990;97(3 Suppl):81-7S. 
|
| 4. | Andziulis A, Gocentas A, Jascaniniene N, Jaszczanin J, Juozulynas A, Radzijewska M. Respiratory function dynamics in individuals with increased motor activity during standard exercise testing. Fiziol Zh 2005;51:80-95. 
|
| 5. | Seaton A, Seaton D, Leitch AG, editors. Crofton and Douglas′s Respiratory Diseases, 5 th ed. Oxford: Oxford University Press: 2000. p. 43-5. 
|
| 6. | Ganong WF. Review of Medical Physiology. 22 nd ed. San Francisco 2005. p. 444. 
|
| 7. | Bonen A, Ling WY, MacIntyre KP, Neil R, McGrail JC, Belcastro AN. Effects of exercise on the serum concentrations of FSH, LH, progesterone and estradiol. Eur J Appl Physiol Occup Physiol. 1979;42:15-23. 
|
| 8. | Jurkowski JE, Jones NL, Walker C, Younglai EV, Sutton JR. Ovarian hormonal responses to exercise. J Appl Physiol Respir Environ Exerc Physiol 1978;44:109-14. 
[PUBMED] |
[Table 1], [Table 2], [Table 3]
|