 |
 |

Preoperative Cardiac Evaluation for Elective Noncardiac Surgery
Darryl Potyk, MD;
Peter Raudaskoski, PharmD, MD
Arch Fam Med. 1998;7:164-173.
ABSTRACT
We reviewed the approach to preoperative cardiac risk assessment, incorporating new information regarding the pathophysiologic features of perioperative myocardial ischemia and recent clinical trials. Relevant articles were identified from a MEDLINE search, followed by bibliography review of the articles identified. The multifactorial risk indexes are valuable in stratifying risks among unselected patients undergoing noncardiac surgery, but they underestimate the risks in selected groups, particularly patients with peripheral vascular disease. The preoperative evaluation of patients with coronary artery disease and risk reduction strategies for high-risk patients are considered. There are no prospective randomized clinical data comparing preoperative revascularization to intensive medical therapy and clinical decisions must be individualized. Risks particular to patients with congestive heart failure and valvular heart disease are also reviewed. Patients with congestive heart failure can undergo noncardiac surgery safely, if their cardiac disease is well-compensated. Patients with aortic stenosis have high risks, and management strategies include valve replacement, aortic valvuloplasty, and aggressive medical treatment. These modalities have not been compared prospectively, and clinical decisions must be individualized. Preoperative arrhythmias are important risk factors, although they appear to confer risk only when due to underlying heart disease. A thorough, targeted history and physical examination supplemented with judicious laboratory studies are usually sufficient to assess a patient's risk for upcoming noncardiac surgery. The clinical history should identify risk factors that predict cardiac complications, and special attention should be given to those risk factors that can be modified before surgery. New developments in perioperative medicine will likely lead to postoperative interventions to reduce silent myocardial ischemia and clinical complications.
INTRODUCTION
Each year, approximately 25 million people in the United States undergo noncardiac surgery. Approximately 8 million of these have cardiac disease or major cardiac risk factors or are older than 65 years. Therefore, it is not surprising that cardiac complications occur when these patients are subjected to stress during the 3- to 4-day perioperative period. Current estimates of serious perioperative cardiac morbidity vary between 1% and 10%, depending on the subset of patients and the type of surgical procedure. About 4% of patients suffer serious perioperative cardiac morbidity following noncardiac surgery.1
Primary care physicians can perform comprehensive preoperative evaluations without subspecialty referral. Preoperative assessment considers the type of surgery (ie, anatomy, fluid shifts, amount of blood loss, and emergency, urgent, or elective procedure), underlying medical illnesses, the pathophysiologic state of the patient, and whether this state can be optimized before surgery. Underlying such an evaluation is an understanding of the physiologic changes that occur with anesthesia. Therefore, we will review the cardiovascular effects of general and regional anesthesia briefly before discussing in more detail the preoperative evaluation for patients with cardiac disease.
Induction of general anesthesia and unconsciousness is achieved with intravenous anesthetics such as propofol.2-4 Once a state of unconsciousness is induced, anesthesia is maintained with inhaled and intravenous anesthetics with or without muscle relaxants. The most common agents used for general anesthesia maintenance are the volatile liquids—halothane, enflurane, isoflurane, sevoflurane, and desflurane.2 Although each of these drugs has unique properties, their cardiovascular effects will be discussed in general terms. The inhaled anesthetic agents reduce arterial blood pressure by decreasing systemic vascular resistance, myocardial contractility, and stroke volume. Patients with a history of congestive heart failure and impaired myocardial contractility are particularly sensitive to drug-induced myocardial depression. The decrease in myocardial contractility can be partially offset by preoperative volume loading via the Frank-Starling mechanism.3, 5-6 The inhaled anesthetics protect the myocardium from ischemic injury. The mechanisms for this protection are unclear, but coronary artery dilatation, diminished myocardial oxygen consumption, and cellular metabolic changes are thought to play significant roles.7-8 However, myocardial ischemia may develop in patients with coronary artery disease and minimal reserves when surgical manipulation activates the sympathetic nervous system, increasing blood pressure, heart rate, and myocardial oxygen consumption.2-3 The inhaled anesthetics can sensitize the myocardium to the effects of circulating catecholamines and increase ventricular irritability and premature ventricular extrasystoles.2-3
Regional anesthetic techniques include spinal, epidural, intravenous, and peripheral nerve blocks. In contrast to general anesthesia, regional anesthesia is not easily reversed once established. Regional techniques occasionally fail to provide adequate analgesia, and general anesthesia is then required.9 Preoperative risk assessment, therefore, must consider the possibility of regional and general anesthesia.
Epidural and spinal techniques produce the greatest physiologic changes. Venodilation occurs with both techniques and can result in decreased preload, decreased cardiac output, and hypotension. Patients with volume depletion and higher-level blocks have the greatest decreases in blood pressure. If the block is sufficiently high (involving T2 to T5), a compensatory tachycardia in response to hypotension does not occur because cardiac sympathetic fibers are blocked. Preoperative volume loading can attenuate the hypotension accompanying regional anesthesia; however, postoperative congestive heart failure may occur as the anesthetic wears off and venous tone returns to normal.2, 10-11
Many clinicians believe that regional is safer than general anesthesia in high-risk patients. There are few data to support this opinion. Prospective randomized studies comparing general and regional anesthesia have shown no differences in mortality, cardiopulmonary complications, or postoperative cognition.12-17 There may, however, be differences that influence the choice of anesthetic technique. Recent studies have suggested improved vessel patency when lower extremity vascular surgery is performed under regional anesthesia.13, 18 Other data suggest the incidence of proximal deep venous thrombosis may be reduced when regional anesthesia is used for lower extremity joint replacement.19-21 Although primary care physicians should have a working knowledge of the physiologic changes that occur during anesthesia to perform a complete preoperative evaluation, the choice of anesthetic technique should be determined by the anesthesiologist.
PREOPERATIVE RISK STRATIFICATION
Preoperative risk stratification depends not only on patient characteristics but also on the proposed surgery. Some operations are more dangerous than others, with risks related to the following 2 important factors: the type of surgery and the degree of hemodynamic stress associated with surgery-specific procedures. High-risk operations include major emergency surgery (particularly in elderly patients), major and peripheral vascular surgery, and other prolonged procedures associated with large amounts of fluid shifts and blood loss. Intermediate-risk procedures include carotid endarterectomy, head and neck procedures, intraperitoneal and intrathoracic, orthopedic, and prostate surgery. Low-risk procedures include endoscopic and superficial procedures and cataract and breast surgery.22
The degree of perioperative risk is further refined when patient-dependent variables are considered. Strategies incorporating clinical variables can identify patients at increased risk for postoperative cardiac complications.23-26 Recent literature has emphasized the relationship between intraoperative and postoperative stress and postoperative cardiac complication.27-30 The value of risk assessment based solely on preoperative risk factors is therefore inherently limited. We will review preoperative risk stratification and new developments in the pathogenesis of postoperative cardiac complications. Although progress has been made in identifying high-risk patients, relatively few data exist regarding risk reduction strategies.31 Where such data are available, we will review them and propose management strategies.
In 1977, Goldman et al23 published a landmark study of a multifactorial index of cardiac risk. Among 1001 consecutive patients undergoing noncardiac surgery, the authors reported 9 variables associated with an increased risk for postoperative cardiac complications (defined as cardiac death, myocardial infarction [MI], pulmonary edema, or ventricular tachycardia). Each risk factor was weighted and assigned a point score, as follows: presence of a third heart sound or jugular venous distention, 11 points; MI within the previous 6 months, 10 points; more than 5 premature ventricular contractions per minute documented at any time before surgery, 7 points; rhythm other than sinus or presence of premature atrial contractions on the preoperative electrocardiogram (ECG), 7 points; being older than 70 years, 5 points; intraperitoneal, intrathoracic, or aortic operation, 3 points; emergency operation, 4 points; significant valvular aortic stenosis (AS) based on results of the physical examination, 3 points; and poor general medical condition, including potassium level of less than 3.0 mmol/L, serum bicarbonate level of less than 20 mmol/L, serum urea nitrogen level of greater than 17.8 mmol/L (50 mg/dL), creatine level of greater than 2287.5 µmol/L (30 mg/dL), PO2 of less than 60 mm Hg, PCO2 of less than 50 mm Hg, elevated results of liver function tests, or any condition causing the patient to be chronically bedridden, 3 points.23 A patient's risk is divided into 4 categories based on their point total (Table 1) This multifactorial index has been validated in prospective studies of unselected patients undergoing general surgical procedures, confirming its usefulness.32-34 In 1986, Detsky et al24-25 revised the original index by changing the point scores for several factors and adding variables, including a history of class III or IV angina, an MI more than 6 months before surgery, and previous pulmonary edema, all of which conferred risk. This revised index score reflects a pretest probability of postoperative complications. Using a nomogram, the pretest probability is combined with a likelihood ratio based on operation-specific complication rates to determine the posttest probability of postoperative complications.24-25 The revised index is useful because it emphasizes that not all surgeries carry the same risk. Although both indexes identify high-risk patients undergoing noncardiac surgery, they both underestimate postoperative complications in patients with significant vascular disease. The cardiac evaluation of patients undergoing major vascular surgery is beyond the scope of this article but has been reviewed elsewhere.35-36 For most patients undergoing elective surgery, a thorough history, physical examination, and ECG are sufficient to assess perioperative cardiac risks.22 Further workup is necessary in few patients (eg, those unable to exercise and those who cannot provide adequate history).
|
|

|
|
Table 1. Goldman Multifactorial Risk Assessment*
|
|
|
High-risk patients should undergo further diagnostic testing only when the results of such tests will change management strategies and lead to improved outcomes.22, 31, 37 Among most ambulatory patients, the test of choice is exercise ECG testing, which provides an estimate of functional capacity and detects myocardial ischemia. In patients with an abnormal resting ECG (eg, left bundle branch block, left ventricular hypertrophy with strain pattern, or digitalis effect), other techniques such as exercise echocardiography or exercise myocardial perfusion imaging should be considered. In patients unable to exercise, a pharmacologic stress test should be used. Dipyridamole thallium and dobutamine hydrochloride echocardiography studies are the most commonly used pharmacologic stress tests.22 A recent meta-analysis showed no differences in the ability of these techniques to predict postoperative cardiac complications.38 Thus, when further testing is necessary, the choice of tests should be determined by patient characteristics and local expertise.22, 39-40
PERIOPERATIVE MYOCARDIAL ISCHEMIA
Continuous ECG monitoring with ST segment analysis has provided insight into the pathophysiologic features and frequency of perioperative myocardial ischemia.29-30 The Perioperative Ischemia Research Group used continuous ECG monitoring before, during, and after noncardiac operations and found that myocardial ischemia occurred most frequently during the early postoperative period (20% in the preoperative, 25% in the intraoperative, and 41% in the postoperative periods). Among a large number of variables, postoperative ischemia had the strongest univariate and the only multivariate associations with clinical cardiac complications.29 Other investigators have confirmed the strong association between postoperative ischemia and clinical cardiac complications.41-43 Postoperative ischemia has been well-characterized. Its peak incidence is within 48 hours of surgery, which correlates with the peak incidence of postoperative MI. The ischemic episodes are not associated with tachycardia and follow a circadian rhythm, with most ischemia occurring in the early morning hours. Postoperative ischemia is more severe than ischemia detected at other times and is clinically silent more than 90% of the time.44-46 Silent postoperative myocardial ischemia has been reported to be present for more than 50 minutes before each clinical event.47 Thus it has been proposed, although not yet studied, that postoperative monitoring of high-risk patients for 48 hours may allow clinicians to intervene and decrease myocardial ischemia. Recent studies have demonstrated that perioperative 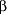 -blocker therapy reduces perioperative ischemia and improves long-term outcomes.48-50 Another study demonstrated that high-dose narcotic analgesia can decrease postoperative ischemia.51 These early results suggest that perioperative treatment of high-risk patients should include -blocker therapy reduces perioperative ischemia and improves long-term outcomes.48-50 Another study demonstrated that high-dose narcotic analgesia can decrease postoperative ischemia.51 These early results suggest that perioperative treatment of high-risk patients should include 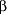 -blockers and aggressive pain control. Postoperative myocardial ischemia is still incompletely understood, and ongoing research will provide further insights into its pathogenesis, prevention, and treatment. -blockers and aggressive pain control. Postoperative myocardial ischemia is still incompletely understood, and ongoing research will provide further insights into its pathogenesis, prevention, and treatment.
CHRONIC STABLE ANGINA
The early studies of perioperative cardiac risk did not identify chronic stable angina as a risk factor for postoperative complications.23 Other studies, however, question this conclusion.52-53 Detsky et al24-25 proposed that chronic stable angina may be associated with an increased risk for complications. Their findings showed that patients with chronic stable angina who were unable to walk 2 blocks at a normal pace (Canadian Cardiovascular Society angina class III and IV) are at increased risk for cardiac complications following noncardiac surgery.24-25 Shah et al53 recently reported that 11% (18/158) of patients with chronic stable angina suffered MI after noncardiac surgery. These patients were not stratified according to their functional status, and cardiac mortality rates were not reported.53 Thus, some patients with chronic stable angina are at increased risk for postoperative cardiac complications. The incidence of complications will vary depending on the patient's preoperative exercise tolerance, surgical procedure, and vigilance with which postoperative cardiac events are searched. Thus, definitive recommendations are difficult to make, and decisions should be individualized. Based on the information available, patients who have good exercise tolerance (able to walk 2 blocks at a normal pace [Canadian Cardiovascular Society angina class I and II]) and a truly stable anginal pattern can undergo surgery safely without further diagnostic workup (Figure 1).
|
|

|
|
Approach to the patient with known or suspected coronary artery disease undergoing elective surgery. For all patients undergoing surgery, antianginal medications should be taken with a sip of water on the morning of surgery; aspirin therapy should be discontinued for 7 to 10 days; and diuretic therapy should be discontinued on the morning of surgery. Asterisk indicates preoperative risk indexes described by Goldman et al23 and modified by Detsky et al24 PTCA, percutaneous transluminal coronary angioplasty; and CABG, coronary artery bypass grafting.
|
|
|
The question of preoperative coronary revascularization for patients with ischemic heart disease is often raised. Coronary artery bypass grafting (CABG) specifically to decrease postoperative complications should be considered differently from a CABG for indications with known survival benefit. There is no question that CABG should be performed before elective noncardiac surgery in patients who have independent indications for coronary revascularization. Whether coronary revascularization specifically decreases postoperative cardiac complications, however, is unclear, and there are few prospective data to guide clinical decisions. Several authors have reported minimal complications in patients who have undergone CABG and then later noncardiac surgery.54-55 In the largest study, 1600 patients enrolled in the Coronary Artery Surgery Study registry who subsequently required noncardiac operations underwent analysis. Patients were separated into the following 3 groups based on findings on their coronary angiograms and their subsequent treatment: those without coronary artery disease, those with coronary artery disease who underwent CABG, and those with significant coronary artery disease who received medical treatment. Surgical mortality following noncardiac operations was then determined for each group. Patients without coronary artery disease had an operative mortality of 0.5%. Patients with significant coronary artery disease who underwent CABG before the noncardiac operation had an operative mortality of 0.9%, and patients with coronary artery disease who received medical treatment had an operative mortality of 2.4%.56 These results are often cited in favor of recommending CABG before noncardiac surgery to decrease perioperative complications. These studies, however, do not address the value of CABG for this indication because of their inherent selection bias. Patients were enrolled when they presented for their second surgical procedure. All patients had survived CABG and were considered candidates for a second major operation. If CABG is recommended specifically to decrease the incidence of surgical complications, morbidity and mortality related to CABG should be included in the overall morbidity and mortality rates. The operative mortality for CABG in the Coronary Artery Surgery Study population was 1.4%.57 Thus, the combination of cardiac and noncardiac surgery may result in a total mortality of 2.3%, which is similar to the perioperative mortality rate associated with noncardiac surgery alone. These mortality estimates are obtained by combining data from different reports (Table 2). Unfortunately, there are no prospective randomized studies to solve this problem. In the absence of prospective data, there is no convincing evidence that prophylactic CABG decreases perioperative morbidity or mortality rates in patients undergoing noncardiac surgery.
|
|

|
|
Table 2. Postoperative Mortality Rates*
|
|
|
Percutaneous transluminal coronary angioplasty (PTCA) has been advocated as an alternative to CABG. It is associated with less morbidity and mortality than CABG and has been recommended before major surgical procedures if significant coronary artery disease is identified. This recommendation is based on anecdotal experience, as there are few data regarding the efficacy of PTCA before noncardiac operations. One report included 148 patients who underwent PTCA and incidentally required noncardiac surgery at a later date. Sixteen cardiac complications, including 1 death, and 7 ischemic events were reported in the postoperative period, for an overall cardiac morbidity of 11%.58 The Mayo Clinic described a retrospective uncontrolled series of 55 patients who underwent PTCA before a noncardiac operation in an attempt to diminish postoperative cardiac morbidity. Their postoperative MI rate was 5.6%, and postoperative mortality was 1.9%.59 The postoperative mortality of 1.9% may not be significantly different from the 2.4% mortality in patients undergoing noncardiac surgery without previous revascularization.56 (Table 2) The PTCA studies are retrospective and without control groups, so it is difficult to assess the effect prophylactic PTCA has on postoperative cardiac complications.
Taking the above information into consideration, the clinical history is of the utmost importance when evaluating a patient with angina before surgery. Assessing whether the patient's ischemic symptoms are truly stable is much more important than extensive diagnostic testing. Assessment of exercise tolerance and the frequency and intensity of anginal symptoms and anginal equivalents should be sought. Self-reported frequency of anginal symptoms, however, can be misleading, because many patients voluntarily reduce their activity level to avoid symptoms.60 Patients whose exercise tolerance has diminished as a result of symptoms cannot be considered to have stable angina and may warrant further evaluation (Figure 1).
UNSTABLE ANGINA
Unstable angina represents an important risk factor for postoperative cardiac complications and is associated with a poor prognosis in patients undergoing CABG and noncardiac surgery.61 A recent series reported that 28% of patients with unstable angina suffered MI after noncardiac surgery.53 Elective noncardiac surgery should be postponed in patients with unstable angina until the ischemia has been stabilized. Medical therapy, coronary catheterization, and revascularization may all be necessary to stabilize the patient. There are no data to indicate the optimal interval between angina stabilization and surgery. If surgery is emergent or urgent, clinical decisions should be individualized weighing the risks and benefits of proceeding. If surgery is elective, an interval of 2 to 3 months seems prudent (Figure 1).
RECENT MI
The patient who has recently suffered an acute MI is at increased risk for postoperative cardiac complications. Early studies reported recurrent MI or cardiac death in 30% of patients undergoing surgery within 3 months of an acute MI and in 15% of patients undergoing noncardiac surgery from 3 to 6 months after an acute MI. After 6 months, the risk for reinfarction or death fell to and remained constant at about 5%.62
Other data suggest that patients with recent MI undergoing noncardiac surgery are at increased risk, but that the risks are not as high as indicated above. Wells and Kaplan63 reported no reinfarctions among 48 patients who had surgery within 3 months of an MI. Using aggressive hemodynamic monitoring during the perioperative period, Rao et al64 reported 6% reinfarction rate among those having surgery within 3 months of an MI, and 2% among those having surgery 3 to 6 months after an MI. The study by Rao et al64 has been criticized because a large proportion of patients underwent minor operations with minimal hemodynamic stress. Shah et al65 recently reported reinfarction rates according to the type of surgery and found that even major operations were associated with reinfarction rates comparable to those reported by Rao et al64 (emergency and vascular surgeries, however, were exceptions and carried higher risks).
These data have led to general recommendations that elective surgery be delayed for 6 months after an acute MI. Risk stratification after an MI, however, allows an individualized approach recognizing the wide clinical spectrum and outcomes after an MI. When surgery is semielective, but prolonged delay may be deleterious (as with a potentially resectable malignant neoplasm), surgery can be considered 4 to 6 weeks after an MI if the patient is at low risk based on results of post-MI exercise testing and left ventricular ejection fraction. Higher-risk patients should be considered for coronary angiography and revascularization before urgent noncardiac surgery. In cases of urgent or emergency surgery, patients with a recent MI should be treated aggressively with 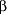 -blockers and invasive hemodynamic monitoring22, 50, 65-66 (Figure 1). -blockers and invasive hemodynamic monitoring22, 50, 65-66 (Figure 1).
CONGESTIVE HEART FAILURE
Goldman et al23 identified congestive heart failure as an important risk factor for postoperative cardiac morbidity and mortality. The decreased myocardial contractility caused by inhaled anesthetics exacerbates the cardiomyopathic state, and it is easy to understand why congestive heart failure predicts postoperative cardiac complications.2-3,23-25 To date, there are no data comparing the perioperative outcomes of patients with diastolic vs systolic dysfunction. This is important because the therapies differ markedly. The following data refer to patients with systolic dysfunction. In the initial study by Goldman et al,23 patients presenting for surgery with a third heart sound or jugular venous distention had a 20% incidence of cardiac death and a 14% incidence of serious cardiac complications. Detsky et al24-25 refined this early work by identifying pulmonary edema within 1 week of surgery and in the remote past as independent risk factors for cardiac events. Charlson et al27 added to these studies by identifying intraoperative blood pressure fluctuations as a risk factor for postoperative congestive heart failure.
Left ventricular ejection fraction determinations have been proposed to identify patients at increased risk for postoperative cardiac complications. As a functional assessment, the ejection fraction was thought to predict how well the heart could withstand the stresses of surgery. Pasternack et al67 reported that patients with ejection fractions of greater than 55% were at low risk for postoperative myocardial complications, whereas those with ejection fractions of less than 35% had a 75% incidence of postoperative MI. More recent studies have suggested that patients with ejection fractions of less than 35% can undergo major surgery with acceptable 30-day mortality rates, whereas others have shown no correlation between ejection fraction and postoperative cardiac complications.68-71 Thus, ejection fraction determinations may supplement the history and physical examination but should not be the primary tool to identify high-risk patients before surgery. The physiologic state of the patient, ie, how well compensated the congestive heart failure is at the time of surgery, is the primary determinant of postoperative outcome. Patients with compensated heart failure (history of heart failure, but presently without jugular venous distention, rales, and a third heart sound) have a 5% to 7% incidence of cardiac complications, while those with decompensated disease have an 18% to 31% incidence of postoperative events.23 Patients with decompensated heart failure should have surgery delayed if possible until their condition is optimized with medical therapy. Detsky et al24-25 indicate these patients should be stabilized for longer than 1 week before undergoing surgery. The optimal interval between an episode of pulmonary edema and elective noncardiac surgery is unclear, although 4 to 6 weeks seems prudent.
The routine use of pulmonary artery catheters in patients with a history of congestive heart failure is controversial.27, 72-74 Patients with stable, well-compensated disease do not require pulmonary artery catheterization, whereas patients with decompensated congestive heart failure and patients undergoing procedures associated with large fluid shifts may benefit from invasive hemodynamic monitoring to guide therapy.
VALVULAR HEART DISEASE
Aortic stenosis has been identified as an important risk factor for postoperative cardiac complications. Hemodynamically significant AS has been associated with a 13% risk for perioperative death.23-25 Aortic stenosis frequently occurs in older individuals who have other serious comorbidities. Because of the increasing prevalence of this lesion, the preoperative assessment of a patient with AS will be considered in detail.
Significant AS should be suspected clinically if the patient has a history of chest pain, syncope, or congestive heart failure or if physical examination reveals the classic murmur with diminished carotid upstrokes.75 When hemodynamically significant AS is suspected, an echocardiogram should be obtained before surgery. A valve area of less than 1.0 cm2 or a gradient of more than 50 mm Hg across the valve suggests that critical AS may be present.
The optimal management of patients with significant AS who require noncardiac surgery has not been defined. Aortic valve replacement is the definitive therapy for patients with symptomatic critical AS being considered for elective surgery. Balloon valvuloplasty of the aortic valve has been advocated as a way to reduce the hemodynamic consequences of AS. Several authors have described small, nonrandomized series of patients with severe AS who underwent percutaneous aortic balloon valvuloplasty before necessary noncardiac surgery. Combining studies, 14 patients underwent successful valvuloplasty (average increase in valve area, 0.25 cm2) followed by noncardiac surgery. Transient intraoperative hypotension requiring pressors developed in 1 patient, but there were no other perioperative complications related to aortic stenosis.76-77 Valvuloplasty carries risks and is associated with a high rate of restenosis, limiting long-term success rates.
Others have questioned the necessity of preoperative valve replacement and valvuloplasty, particularly for patients with minimal or no symptoms. A heightened awareness of the potential risks for patients with AS undergoing surgery, together with improved diagnostic capabilities and advanced anesthetic management techniques, may enable these patients to undergo surgery safely without other interventions. O'Keefe et al78 described a series of 23 patients with severe AS (mean gradient, 76 mm Hg; mean valve area, 0.61 cm2) who underwent elective noncardiac surgery under general or spinal anesthesia. There was no cardiac mortality among this group, but transient hypotension developed in 5 patients, who responded to fluids and pressors. The authors concluded that selected patients with severe AS can undergo noncardiac surgery with acceptable risks if invasive hemodynamic monitoring and vigilant anesthetic management techniques are used.78 Although aortic valve replacement before noncardiac surgery is preferred when feasible, situations arise in which this is not possible (urgent or emergency surgery or the patient refuses or is not a candidate). Under these circumstances, aortic valvuloplasty can be considered as a temporizing measure, but it is not clear whether balloon valvuloplasty or aggressive anesthetic management alone results in fewer complications. In the absence of prospective comparative data, clinical decisions should be individualized after considering the nature and urgency of the problem and after discussions with the patient, surgeon, and anesthesiologist.
Patients with mitral stenosis require elevated filling pressures and adequate filling time to ensure adequate left ventricular volume and cardiac output. Symptoms of significant mitral stenosis include dyspnea on exertion and congestive heart failure. The hemodynamic changes associated with anesthesia and surgery, particularly tachycardia, can result in pulmonary edema and cardiogenic shock. An echocardiogram should be obtained for symptomatic patients. Mitral valve replacement should be considered for symptomatic patients with a valve area of less than 1.0 cm2. Pulmonary artery catheterization may be helpful in these patients if large volume shifts are anticipated, and the anesthesiologist should be alerted of the possible need for negative chronotropic drugs to treat tachycardias resulting in hemodynamic compromise.22
The left ventricle in patients with chronic aortic insufficiency or mitral regurgitation is subjected to high volume loads that in time result in impaired myocardial contractility. Patients with regurgitant lesions are not as sensitive to the subtle hemodynamic shifts that affect patients with stenotic lesions. If patients with valvular insufficiency do not have signs or symptoms of congestive heart failure, they can undergo noncardiac surgery safely, usually without invasive hemodynamic monitoring.22 Those with mild to moderate left ventricular dysfunction should receive medical treatment and delay surgery until after 4 to 6 weeks of optimization. Pulmonary artery catheterization may be considered if large fluid shifts or significant loss of blood is expected. In patients with severe left ventricular dysfunction, valve replacement and lesser or alternative surgical options should be considered.
Patients with prosthetic heart valves and those with atrial fibrillation often use oral anticoagulants. These patients are at risk for thromboembolic complications when anticoagulation therapy is discontinued during the perioperative period. The risks for bleeding and thrombosis must be balanced, and anticoagulation therapy should be discontinued for as brief a time as possible. The risk-benefit assessment should include the type of valve, position, cardiac rhythm, and history. Ball-cage prosthetic heart valves (Starr-Edwards type) or older, pivoting disk valves (Bjork-Shiley type) are more thrombogenic than newer valves (St Judes type). Valves in the mitral position are associated with more thromboembolic complications than those in the aortic position.79-80 Atrial fibrillation is also associated with increased thrombotic risk, particularly when associated with mitral valve disease.80-81 Patients with a history of embolic episodes are also at increased risk for recurrent events.80-81 Although some evidence suggests that minor procedures such as dental surgery and cataract surgery can be performed without discontinuing anticoagulation therapy, most procedures require interruption of therapy to avoid excessive bleeding.82-83 The international normalized ratio (INR) decreases exponentially beginning 24 to 36 hours after the last warfarin sodium dose, with a slower rate of decline in the elderly. The risk for perioperative thrombotic complications with a normal INR has not been defined, nor has the preoperative INR value associated with the lowest risk for excessive operative bleeding. The optimal INR at the time of surgery is likely to vary according to the type of surgery (variables include accessible site, enclosed space [ie, central nervous system or the spine], and vital structure [ie, retina]) and the anticipated amount of blood loss. Assuming that an INR of less than 1.5 is associated with an acceptable risk for perioperative bleeding, patients with an INR of 2.0 to 3.0 will have an INR of less than 1.5 approximately 4 to 5 days after discontinuation of warfarin therapy.84 Therefore, warfarin therapy should be discontinued 4 to 5 days before elective surgery (longer for elderly individuals). Following surgery, warfarin or heparin sodium therapy can be restarted, the choice depending on the risk for thrombosis. Patients at high risk for thrombosis should be admitted for intravenous heparin therapy while the INR is falling. These patients should have the heparin therapy discontinued 6 hours before surgery and then restarted postoperatively.85-88
Questions regarding the need for prophylactic antibiotics to prevent bacterial endocarditis are common. Although this is an important topic, a complete discussion of endocarditis prophylaxis is beyond our scope. Excellent reviews are available, and primary care physicians should be familiar with the American Heart Association recommendations for endocarditis prophylaxis.89-90 All patients with valvular lesions should be considered for bacterial endocarditis prophylaxis.
ARRHYTHMIAS
Patients with preoperative arrhythmias have been described as experiencing increased perioperative morbidity and mortality. Five or more premature ventricular contractions per minute at any time or nonsinus rhythm or premature atrial contractions detected on the preoperative ECG are associated with increased perioperative cardiovascular morbidity and death.23-25,52 In one study, life-threatening cardiac complications occurred in 16% of patients (7/44) with frequent premature ventricular contractions and in 10% of patients (11/112) with premature atrial contractions or nonsinus rhythm on the preoperative ECG.23 A more recent investigation,91 however, questions these results. Among 230 high-risk patients with continuous ECG monitoring, no association between preoperative ventricular arrhythmias and perioperative cardiac complications could be demonstrated.91 The different conclusions reached by these studies are not surprising when the results are examined in more detail. Even in the studies reporting an association between preoperative arrhythmias and postoperative cardiac events, the complications were not caused by arrhythmias. The increased morbidity and mortality were consistently due to myocardial ischemic events and postoperative congestive heart failure. The incidence of perioperative cardiac events appears to be more closely related to underlying heart disease than to preoperative arrhythmias.1, 23, 52, 91-92 Therefore, the preoperative evaluation of the patient with arrhythmias should be directed toward determining the cause, diagnosing underlying cardiac disease, and detecting the presence of other perioperative risk factors. A thorough history and physical examination directed at the cardiac and pulmonary systems, together with ECG and laboratory studies to detect electrolyte or acid-base disorders, are usually sufficient.22
CONDUCTION DISTURBANCES AND PACEMAKERS
Patients with underlying conduction disturbances and permanent pacemakers require a meticulous preoperative evaluation. Patients with conduction disturbances should undergo evaluation for other signs and symptoms of underlying cardiac disease. Perioperative management is determined primarily by the underlying heart disease. The indications for temporary pacemakers during the perioperative period are generally the same as those for permanent pacemakers.93 Patients with preexisting bundle branch block or intraventricular conduction delays generally require no special precautions in the perioperative period. An exception is the patient with left bundle branch block for whom a pulmonary artery catheter is thought to be necessary. Under these circumstances, an external pacemaker or a temporary transvenous pacing catheter should be available to treat complete heart block that may result from the presence of the catheter.94 Bifascicular block does not progress to complete heart block more frequently in the perioperative period than at other times, and prophylactic pacing is not indicated.95
Patients with permanent pacemakers can undergo surgery safely if precautions are taken. The use of electrocautery represents a significant risk to these patients. The electrical stimulus from electrocautery may inhibit demand pacemakers, may be conducted down the lead to the heart and cause ventricular tachycardia, or may reprogram the pacemaker. These problems can be avoided by positioning the ground plate for the electrical circuit so that the electrical current travels away from the generator by keeping the electrocautery device away from the pacemaker generator as much as possible and by using the electrocautery only in short bursts. The pacemaker should be set in an asynchronous or nonsensing mode in patients who are pacemaker dependent and whose underlying rhythm is unreliable. The asynchronous mode can be achieved through reprogramming or by placing a magnet over the pacemaker generator. The pacemaker should be interrogated after surgery to ensure appropriate programming and sensing-pacing thresholds.96-98
CONCLUSION
Evaluation of patients with cardiovascular disease before elective noncardiac surgery is a challenge. Primary care physicians should be able to evaluate most perioperative risks without subspecialty referral. A thorough, targeted history and physical examination supplemented with judicious laboratory studies are usually sufficient to adequately assess a patient's risks for upcoming surgery. The clinical history should identify risk factors that predict cardiac complications, and special attention should be given to those risk factors that can be modified before surgery. Primary care physicians should follow up their patients postoperatively, with a heightened awareness of postoperative myocardial ischemia, its peak incidence, and its atypical presentations. New developments in perioperative medicine will likely lead to postoperative interventions to reduce silent myocardial ischemia and clinical complications.
Since the manuscript was accepted for publication, another review of the literature and guidelines for assessing and managing perioperative risk due to coronary artery disease associated with major noncardiac surgery have been published by the American College of Physicians.99-100 Our recommendations are consistent with these guidelines.
AUTHOR INFORMATION
Accepted for publication March 19, 1997.
We thank R. Jon Auricchio, PharmD, for his thoughtful comments and review of the manuscript.
Reprints: Darryl Potyk, MD, Internal Medicine Spokane, 101 W Eighth Ave, PO Box 2555, Spokane, WA 99220-2555.
From Internal Medicine Spokane, Internal Medicine Residency Program, affiliate of the Department of Medicine, University of Washington School of Medicine, Spokane (Dr Potyk); and the Department of Anesthesia, Alvarado Hospital (Dr Raudaskoski), and Anesthesia Services Medical Group (Dr Raudaskoski), San Diego, Calif.
REFERENCES
1. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72:153-184.
WEB OF SCIENCE
| PUBMED
2. Appleby J, Lawrence VA. Anesthesia. J Gen Intern Med. 1994;9:635-647.
WEB OF SCIENCE
| PUBMED
3. Stoelting RK, Miller RD. Effects of inhaled anesthetics on ventilation and circulation. In: Basics of Anesthesia. 2nd ed. New York, NY: Churchill Livingstone Inc; 1989:43-56.
4. Larijani GE, Gratz I, Afshar M, Jacobi AG. Clinical pharmacology of propofol: an intravenous anesthetic agent. Ann Pharmacother. 1989;23:743-749.
ABSTRACT
5. Kemmotsu O, Hashimoto Y, Shimosato S. The effects of fluroxene and enflurane on contractile performance of isolated papillary muscles from failing hearts. Anesthesiology. 1974;40:252-260.
WEB OF SCIENCE
| PUBMED
6. Fyman PN, Cottrell JE, Kushins L, Casthely PA. Vasodilator therapy in the perioperative period. Can J Anaesth. 1986;33:629-643.
7. Coetzee A, Brits W, Genade S, Lochner A. Halothane does have protective properties in the isolated ischemic rat heart. Anesth Analg. 1991;73:711-719.
FREE FULL TEXT
8. Kersten JR, Schmeling TJ, Hettrick DA, Pagel PS, Gross GJ, Warltier DC. Mechanism of myocardial protection by isoflurane: role of adenosine triphosphate-regulated potassium channels. Anesthesiology. 1996;85:794-807.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
9. Levy JH, Islas JA, Ghia JN, Turnbull C. A retrospective study of the incidence and causes of failed spinal anesthetics in a university hospital. Anesth Analg. 1985;64:705-710.
FREE FULL TEXT
10. Stoelting RK, Miller RD. Spinal, epidural, and caudal blocks. In: Basics of Anesthesia. 2nd ed. New York, NY: Churchill Livingstone Inc; 1989:173-187.
11. Underwood SM, Glynn CJ. Sick sinus syndrome manifest after spinal anaesthesia. Anaesthesia. 1988;43:307-309.
WEB OF SCIENCE
| PUBMED
12. Bode RH, Lewis KP, Zarich SW, et al. Cardiac outcome after peripheral vascular surgery: comparison of general and regional anesthesia. Anesthesiology. 1996;84:3-13.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
13. Christopherson R, Beattie C, Frank SM, et al. Perioperative morbidity in patients randomized to epidural or general anesthesia for lower extremity vascular surgery. Anesthesiology. 1993;79:422-434.
WEB OF SCIENCE
| PUBMED
14. Clark JL. Epidural anesthesia and analgesia in high risk surgical patients. Anesthesiology. 1987;67:1025-1026.
15. Scott NB, Kehlet H. Regional anesthesia and surgical morbidity. Br J Surg. 1988;75:299-304.
WEB OF SCIENCE
| PUBMED
16. Williams-Russo P, Mattis S, Szatrowski TP, Sharrock NE, Charlson ME. Cognitive effects after epidural vs general anesthesia in older adults. JAMA. 1995;274:44-50.
FREE FULL TEXT
17. Yeager MP, Glass DD, Neff RK, Brinck-Johnsen T. Epidural anesthesia and analgesia in high-risk surgical patients. Anesthesiology. 1987;66:729-736.
WEB OF SCIENCE
| PUBMED
18. Rosenfield BA, Beattie C, Christopherson R, et al. The effects of different anesthetic regimens on fibrinolysis and the development of postoperative arterial thrombosis. Anesthesiology. 1993;79:435-443.
WEB OF SCIENCE
| PUBMED
19. Mitchell D, Friedman RJ, Baker JD, Cooke JE, Darcy MD, Miller MC. Prevention of thromboembolic disease following total knee arthroplasty: epidural versus general anesthesia. Clin Orthop. 1991;269:109-112.
20. Nielsen PT, Jorgensen LN, Albrecht-Beste E, Leffers AM, Rasmussen LS. Lower thrombosis risk with epidural blockade in knee arthroplasty. Acta Orthop Scand. 1990;61:29-31.
WEB OF SCIENCE
| PUBMED
21. Modig J, Maripuu E, Sahlsedt B. Thromboembolism following total hip replacement: a prospective investigation of 94 patients with emphasis on the efficacy of lumbar epidural anesthesia in prophylaxis. Reg Anesth. 1986;11:72-79.
22. Committee on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. Guidelines for perioperative cardiovascular evaluation for noncardiac surgery: report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol. 1996;27:910-948.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
23. Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845-850.
WEB OF SCIENCE
| PUBMED
24. Detsky AS, Abrams HB, McLaughlin JR, et al. Predicting cardiac complications in patients undergoing noncardiac surgery. J Gen Intern Med. 1986;1:211-219.
WEB OF SCIENCE
| PUBMED
25. Detsky AS, Abrams HB, Forbath N, Scott JG, Hilliard JR. Cardiac assessment for patients undergoing noncardiac surgery: a multifactorial clinical risk index. Arch Intern Med. 1986;146:2131-2134.
FREE FULL TEXT
26. Larsen SF, Olesen KH, Jacobsen E, et al. Prediction of cardiac risk in non-cardiac surgery. Eur Heart J. 1987;8:179-185.
FREE FULL TEXT
27. Charlson ME, MacKenzie CR, Gold JP, Ales KL, Topkins M, Shires GT. Risk for postoperative congestive heart failure. Surg Gynecol Obstet. 1991;172:95-104.
WEB OF SCIENCE
| PUBMED
28. Mauney FM, Ebert PA, Sabiston DC Jr. Postoperative myocardial infarction: a study of predisposing factors, diagnosis and mortality in a high risk group of surgical patients. Ann Surg. 1970;172:497-503.
WEB OF SCIENCE
| PUBMED
29. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery: The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323:1781-1788.
WEB OF SCIENCE
| PUBMED
30. Raby KE, Goldman L, Creager MA, et al. Correlation between preoperative ischemia and major cardiac events after peripheral vascular surgery. N Engl J Med. 1989;321:1296-1300.
WEB OF SCIENCE
| PUBMED
31. Massie BM, Mangano DT. Risk stratification for noncardiac surgery: how (and why)? Circulation. 1993;87:1752-1755.
FREE FULL TEXT
32. Zeldin RA. Assessing cardiac risk in patients who undergo noncardiac surgical procedures. Can J Surg. 1984;27:402-404.
WEB OF SCIENCE
| PUBMED
33. Jeffrey CC, Kunsman J, Cullen DJ, Brewster DC. A prospective evaluation of cardiac risk index. Anesthesiology. 1983;58;462-464.
34. Goldman L. Multifactorial index of cardiac risk in noncardiac surgery: ten-year status report. J Cardiothorac Anesth. 1987;1:237-244.
FULL TEXT
| PUBMED
35. Wong T, Detsky AS. Preoperative cardiac risk assessment for patients having peripheral vascular surgery. Ann Intern Med. 1992;116:743-753.
36. Potyk DK. Cardiac evaluation and risk reduction in patients undergoing major vascular operations. West J Med. 1994;161:50-56.
WEB OF SCIENCE
| PUBMED
37. Goldman L. Assessment of perioperative risk. N Engl J Med. 1994;330:707-709.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
38. Mantha S, Roizen MF, Barnard J, Thisted RA, Ellis JE, Foss J. Relative effectiveness of four preoperative tests for predicting adverse cardiac outcome after vascular surgery: a meta-analysis. Anesth Analg. 1994;79:422-433.
FREE FULL TEXT
39. Mangano DT, Goldman L. Preoperative assessment of patients with known or suspected coronary disease. N Engl J Med. 1995;333:1750-1756.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
40. Mayo Clinic Cardiovascular Working Group on Stress Testing. Cardiovascular stress testing: a description of the various types of stress tests and indications for their use. Mayo Clin Proc. 1996;71:43-52.
FREE FULL TEXT
41. Ouyang P, Gerstenblith G, Furman WR, Golueke PJ, Gottleib SO. Frequency and significance of early postoperative silent myocardial ischemia in patients having peripheral vascular surgery. Am J Cardiol. 1989;64:1113-1116.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
42. Hollenberg M, Mangano DT, Browner WS, London MJ, Tubau JF, Tateo IM. Predictors of postoperative myocardial ischemia in patients undergoing noncardiac surgery: The Study of Perioperative Ischemia Research Group. JAMA. 1992;268:205-209.
FREE FULL TEXT
43. McCann RL, Clements FM. Silent myocardial ischemia in patients undergoing peripheral vascular surgery: incidence and association with perioperative cardiac morbidity and mortality. J Vasc Surg. 1989;9:583-587.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
44. Mangano DT, Hollenberg M, Fegert G, et al. Perioperative myocardial ischemia in patients undergoing noncardiac surgery, I: incidence and severity during the 4 day perioperative period. J Am Coll Cardiol. 1991;17:843-850.
ABSTRACT
45. Mangano DT, Wong MG, London MJ, Tubau JF, Rapp JA. Perioperative myocardial ischemia in patients undergoing noncardiac surgery, II: incidence and severity during the first week after surgery. J Am Coll Cardiol. 1991;17:851-857.
ABSTRACT
46. Marsch SCU, Schaefer HG, Skarvan K, Castelli F, Scheidegger D. Perioperative myocardial ischemia in patients undergoing elective hip arthroplasty during lumbar regional anesthesia. Anesthesiology. 1992;76:518-527.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
47. Raby KE, Barry J, Creager MA, Cook EF, Weisberg MC, Goldman L. Detection and significance of intraoperative and postoperative myocardial ischemia in peripheral vascular surgery. JAMA. 1992;268:222-227.
FREE FULL TEXT
48. Imperi GA, Lambert CR, Coy K, Lopez L, Pepine CT. Effects of titrated 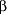 -blockade (metoprolol) on silent myocardial ischemia in ambulatory patients with coronary artery disease. Am J Cardiol. 1987;60:519-524.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
49. Pasternack PF, Imparato AM, Baumann FG, et al. The hemodynamics of -blockade (metoprolol) on silent myocardial ischemia in ambulatory patients with coronary artery disease. Am J Cardiol. 1987;60:519-524.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
49. Pasternack PF, Imparato AM, Baumann FG, et al. The hemodynamics of 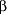 -blockade in patients undergoing abdominal aortic aneurysm repair. Circulation. 1987;76(pt 3):1-7.
50. Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. N Engl J Med. 1996;335:1713-1720.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
51. Siliciano D, Hollenberg M, Goehner P, Mangano D. Use of continuous versus intermittent narcotic after CABG surgery: effects on myocardial ischemia. Anesth Analg. 1990;70(suppl):S371.
52. Cooperman M, Pflug B, Martin EW, Evans WE. Cardiovascular risk factors in patients with peripheral vascular disease. Surgery. 1978;84:505-509.
WEB OF SCIENCE
| PUBMED
53. Shah KB, Kleinman BS, Rao TLK, Jacobs HK, Mestan K, Schaafsma M. Angina and other risk factors in patients with cardiac diseases undergoing noncardiac operations. Anesth Analg. 1990,70:240-247.
54. Reul GJ, Cooley DA, Duncan JM, et al. The effect of coronary bypass on the outcome of peripheral vascular operations in 1093 patients. J Vasc Surg. 1986;3:788-798.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
55. Toal KW, Jacocks MA, Elkins RC. Preoperative coronary bypass grafting in patients undergoing abdominal aortic aneurysm reconstruction. Am J Surg. 1984;148:825-829.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
56. Foster ED, Davis KB, Carpenter JA, Abele S, Fray D. Risk of noncardiac operation in patients with defined coronary disease: The Coronary Artery Surgery Study (CASS) registry experience. Ann Thorac Surg. 1986;41:42-50.
FREE FULL TEXT
57. Principal Investigators of CASS and Their Associates. The National Heart, Lung, and Blood Institute Coronary Artery Surgery Study. Circulation. 1981;63(suppl)I1-I81.
58. Allen JR, Helling TS, Hartzler GO. Operative procedures not involving the heart after percutaneous transluminal coronary angioplasty. Surg Gynecol Obstet. 1991;173:285-288.
WEB OF SCIENCE
| PUBMED
59. Huber KC, Evans MA, Bresnahan JF, Gibbons RJ, Holmes DR. Outcome of noncardiac operations in patients with severe coronary artery disease successfully treated preoperatively with coronary angioplasty. Mayo Clin Proc. 1992;67:15-21.
WEB OF SCIENCE
| PUBMED
60. Goldman L, Cook EF, Mitchell N, Flatley M, Sherman H, Cohn PF. Pitfalls in the serial assessment of cardiac functional status: how a reduction in "ordinary" activity may reduce the apparent degree of cardiac compromise and give a misleading impression of improvement. J Chronic Dis. 1982;35:763-771.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
61. Russell RO, Resnekov L, Wolk M, et al. Unstable angina pectoris: national cooperative study group to compare surgical and medical therapy, II: in hospital experience and initial follow-up results in patients with one, two, and three vessel disease. Am J Cardiol. 1978:42:839-848.
62. Goldman L. Cardiac risks and complications of noncardiac surgery. Ann Intern Med. 1983;98:504-513.
63. Wells PH, Kaplan JA. Optimal management of patients with ischemic heart disease for noncardiac surgery by complementary anesthesiologist and cardiologist interaction. Am Heart J. 1981;102(6 pt 1):1029-1037.
64. Rao TLK, Jacobs KH, El-Etra AA. Reinfarction following anesthesia in patients with myocardial infarction. Anesthesiology. 1983;59:499-505.
WEB OF SCIENCE
| PUBMED
65. Shah KB, Kleinman BS, Sami H, Patel J, Rao TLK. Reevaluation of perioperative myocardial infarction in patients with prior myocardial infarction undergoing noncardiac operations. Anesth Analg. 1990;71:231-235.
FREE FULL TEXT
66. Weitz HH, Goldman L. Noncardiac surgery in the patient with heart disease. Med Clin North Am. 1987;71:413-432.
WEB OF SCIENCE
| PUBMED
67. Pasternack PF, Imparato AM, Riles TS, et al. The value of the radionuclide angiogram in the prediction of perioperative myocardial infarction in patients undergoing lower extremity revascularization procedures. Circulation. 1985;72(part 2):3-7.
68. Halm EA, Browner WS, Tubau JF, Tateo IM, Mangano DT. Echocardiography for assessing cardiac risk in patients having noncardiac surgery. Ann Intern Med. 1996;125:433-441.
FREE FULL TEXT
69. Lazor L, Russell JC, DaSilva J, Radford M. Use of the multiple uptake gated acquisition scan for the preoperative assessment of cardiac risk. Surg Gynecol Obstet. 1988;167:234-238.
WEB OF SCIENCE
| PUBMED
70. Franco CD, Goldsmith J, Veith FJ, et al. Resting gated pool ejection fraction: a poor predictor of perioperative myocardial infarction in patients undergoing vascular surgery for infrainguinal bypass grafting. J Vasc Surg. 1989;10:656-661.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
71. Kazmers A, Cerqueira MD, Zierler RE. Perioperative and late outcome in patients with left ventricular ejection fraction of 35% or less who require major vascular surgery. J Vasc Surg. 1988;8:307-315.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
72. Davies MJ, Cronin KD, Domaingue CM. Pulmonary artery catheterisation: an assessment of risks and benefits in 220 surgical patients. Anaesth Intens Care. 1982:10:9-14.
73. Berlauk JF, Abrams JH, Gilmour IJ, O'Connor SR, Knighton DR, Cerra FB. Preoperative optimization of cardiovascular hemodynamics improves outcome in peripheral vascular surgery: a prospective randomized trial. Ann Surg. 1991;214:289-299.
WEB OF SCIENCE
| PUBMED
74. Whittemore AD, Clowes AW, Hechtman HB, Mannick JA. Aortic aneurysm repair: reduced operative mortality associated with maintenance of optimal cardiac performance. Ann Surg. 1980;192:414-421.
WEB OF SCIENCE
| PUBMED
75. Perloff JF. Clinical recognition of aortic stenosis: the physical signs and differential diagnosis of the various signs of obstruction to left ventricular outflow. Prog Cardiovasc Dis. 1968;10:323-330.
FULL TEXT
76. Roth RB, Palacios IF, Block PC. Percutaneous aortic balloon valvuloplasty: its role in the management of patients with aortic stenosis requiring major noncardiac surgery. J Am Coll Cardiol. 1989;13:1039-1041.
ABSTRACT
77. Levine MJ, Berman AD, Safian RD, Diver DJ, McKay RG. Palliation of valvular aortic stenosis by balloon valvuloplasty as preoperative preparation for noncardiac surgery. Am J Cardiol. 1988;62:1309-1310.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
78. O'Keefe JH, Shub C, Rettke SR. Risk of noncardiac surgical procedures in patients with aortic stenosis. Mayo Clin Proc. 1989;64:400-405.
WEB OF SCIENCE
| PUBMED
79. Hammermeister KE, Henderson WG, Burchfiel CM, et al. Comparison of outcome after valve replacement with a bioprosthesis versus a mechanical prosthesis: initial 5 year results in a randomized trial. J Am Coll Cardiol. 1987;10:719-732.
ABSTRACT
80. Edmunds LH. Thromboembolic complications of current cardiac valvular prostheses. Ann Thorac Surg. 1982;34:96-106.
FREE FULL TEXT
81. The Stroke Prevention in Atrial Fibrillation Investigators. Predictors of thromboembolism in atrial fibrillation, I: clinical features of patients at risk. Intern Med. 1992;116:1-5.
82. Gainey SP, Robertson DM, Fay W, Ilstrup D. Ocular surgery on patients receiving long-term warfarin therapy. Am J Ophthalmol. 1989;108:142-146.
WEB OF SCIENCE
| PUBMED
83. Ramstrom G, Sindet-Pedersen S, Hall G, Blomback M, Alander U. Prevention of postsurgical bleeding in oral surgery using tranexamic acid without dose modification of oral anticoagulants. J Oral Maxillofac Surg. 1993;51:1211-1216.
WEB OF SCIENCE
| PUBMED
84. White RH, McKittrick T, Hutchinson R, Twitchell J. Temporary discontinuation of warfarin therapy: changes in the international normalized ratio. Ann Intern Med. 1995;122:40-42.
FREE FULL TEXT
85. Stein PD, Alpert JS, Copeland J, Dalen JE, Goldman S, Turpie AGG. Antithrombotic therapy in patients with mechanical and biological prosthetic heart valves. Chest. 1992;102(suppl):445S-455S.
86. Travis S, Wray R, Harrison K. Perioperative anticoagulant control. Br J Surg. 1989;76:1107-1108.
WEB OF SCIENCE
| PUBMED
87. Tinker JH, Tarhan S. Discontinuing anticoagulant therapy in surgical patients with cardiac valve prostheses: observations in 180 operations. JAMA. 1978;239:738-739.
FREE FULL TEXT
88. Katholi RE, Nolan SP, McGuire LB. The management of anticoagulation during noncardiac operations in patients with prosthetic heart valves: a prospective study. Am Heart J. 1978;96:163-165.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
89. Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis: recommendations by the American Heart Association. JAMA. 1997;277:1794-1801.
FREE FULL TEXT
90. Brown FH. Prevention of bacterial endocarditis. In: Goldman DR, Brown FH, Guarnieri DM, eds. Perioperative Medicine. New York, NY: McGraw-Hill Book Co; 1994:421-434.
91. O'Kelly B, Browner WS, Massie B, Tubau J, Ngo L, Mangano DT. Ventricular arrhythmias in patients undergoing noncardiac surgery. JAMA. 1992;268:217-221.
FREE FULL TEXT
92. Vanik PE, Davis HS. Cardiac arrhythmias during halothane anesthesia. Anesth Analg. 1968;47:299-307.
FREE FULL TEXT
93. Committee on Pacemaker Implantation. Guidelines for implantation of cardiac pacemakers and antiarrhythmia devices: a report of the American College of Cardiology/American Heart Association Task Force on Assessement of Diagnostic and Therapeutic Cardiovascular Procedures (Committee on Pacemaker Implantation). Circulation. 1991;84:455-467.
FREE FULL TEXT
94. Berliner D, Okun M, Peters RW, Carliner NH, Plotnick GD, Fisher ML. Transcutaneous temporary pacing in the operating room. JAMA. 1985;254:84-86.
FREE FULL TEXT
95. Bellocci F, Santarelli P, DiGennaro M, Ansalone G, Fenici R. The risk of cardiac complications in surgical patients with bifascicular block: a clinical and electrophysiologic study in 98 patients. Chest. 1980;77:343-348.
FREE FULL TEXT
96. Erdman S, Levinsky L, Servadio C, Stoupel E, Levy MJ. Safety precautions in the management of patients with pacemakers when electrocautery operations are performed. Surg Gynecol Obstet. 1988;167:311-314.
WEB OF SCIENCE
| PUBMED
97. Shapiro WA, Roizen MF, Singleton MA, Morady F, Bainton CR, Gaynor RL. Intraoperative pacemaker complications. Anesthesiology. 1985;63:319-322.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
98. Simon A. Perioperative management of the pacemaker patient. Anesthesiology. 1977;46:127-131.
WEB OF SCIENCE
| PUBMED
99. American College of Physicians. Guidelines for assessing and managing the perioperative risk from coronary artery disease associated with major noncardiac surgery. Ann Intern Med. 1997;127:309-312.
FREE FULL TEXT
100. Palda VA, Detsky AS. Perioperative assessment and management risk from coronary artery disease. Ann Intern Med. 1997;127:313-328.
FREE FULL TEXT -blockade in patients undergoing abdominal aortic aneurysm repair. Circulation. 1987;76(pt 3):1-7.
50. Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. N Engl J Med. 1996;335:1713-1720.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
51. Siliciano D, Hollenberg M, Goehner P, Mangano D. Use of continuous versus intermittent narcotic after CABG surgery: effects on myocardial ischemia. Anesth Analg. 1990;70(suppl):S371.
52. Cooperman M, Pflug B, Martin EW, Evans WE. Cardiovascular risk factors in patients with peripheral vascular disease. Surgery. 1978;84:505-509.
WEB OF SCIENCE
| PUBMED
53. Shah KB, Kleinman BS, Rao TLK, Jacobs HK, Mestan K, Schaafsma M. Angina and other risk factors in patients with cardiac diseases undergoing noncardiac operations. Anesth Analg. 1990,70:240-247.
54. Reul GJ, Cooley DA, Duncan JM, et al. The effect of coronary bypass on the outcome of peripheral vascular operations in 1093 patients. J Vasc Surg. 1986;3:788-798.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
55. Toal KW, Jacocks MA, Elkins RC. Preoperative coronary bypass grafting in patients undergoing abdominal aortic aneurysm reconstruction. Am J Surg. 1984;148:825-829.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
56. Foster ED, Davis KB, Carpenter JA, Abele S, Fray D. Risk of noncardiac operation in patients with defined coronary disease: The Coronary Artery Surgery Study (CASS) registry experience. Ann Thorac Surg. 1986;41:42-50.
FREE FULL TEXT
57. Principal Investigators of CASS and Their Associates. The National Heart, Lung, and Blood Institute Coronary Artery Surgery Study. Circulation. 1981;63(suppl)I1-I81.
58. Allen JR, Helling TS, Hartzler GO. Operative procedures not involving the heart after percutaneous transluminal coronary angioplasty. Surg Gynecol Obstet. 1991;173:285-288.
WEB OF SCIENCE
| PUBMED
59. Huber KC, Evans MA, Bresnahan JF, Gibbons RJ, Holmes DR. Outcome of noncardiac operations in patients with severe coronary artery disease successfully treated preoperatively with coronary angioplasty. Mayo Clin Proc. 1992;67:15-21.
WEB OF SCIENCE
| PUBMED
60. Goldman L, Cook EF, Mitchell N, Flatley M, Sherman H, Cohn PF. Pitfalls in the serial assessment of cardiac functional status: how a reduction in "ordinary" activity may reduce the apparent degree of cardiac compromise and give a misleading impression of improvement. J Chronic Dis. 1982;35:763-771.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
61. Russell RO, Resnekov L, Wolk M, et al. Unstable angina pectoris: national cooperative study group to compare surgical and medical therapy, II: in hospital experience and initial follow-up results in patients with one, two, and three vessel disease. Am J Cardiol. 1978:42:839-848.
62. Goldman L. Cardiac risks and complications of noncardiac surgery. Ann Intern Med. 1983;98:504-513.
63. Wells PH, Kaplan JA. Optimal management of patients with ischemic heart disease for noncardiac surgery by complementary anesthesiologist and cardiologist interaction. Am Heart J. 1981;102(6 pt 1):1029-1037.
64. Rao TLK, Jacobs KH, El-Etra AA. Reinfarction following anesthesia in patients with myocardial infarction. Anesthesiology. 1983;59:499-505.
WEB OF SCIENCE
| PUBMED
65. Shah KB, Kleinman BS, Sami H, Patel J, Rao TLK. Reevaluation of perioperative myocardial infarction in patients with prior myocardial infarction undergoing noncardiac operations. Anesth Analg. 1990;71:231-235.
FREE FULL TEXT
66. Weitz HH, Goldman L. Noncardiac surgery in the patient with heart disease. Med Clin North Am. 1987;71:413-432.
WEB OF SCIENCE
| PUBMED
67. Pasternack PF, Imparato AM, Riles TS, et al. The value of the radionuclide angiogram in the prediction of perioperative myocardial infarction in patients undergoing lower extremity revascularization procedures. Circulation. 1985;72(part 2):3-7.
68. Halm EA, Browner WS, Tubau JF, Tateo IM, Mangano DT. Echocardiography for assessing cardiac risk in patients having noncardiac surgery. Ann Intern Med. 1996;125:433-441.
FREE FULL TEXT
69. Lazor L, Russell JC, DaSilva J, Radford M. Use of the multiple uptake gated acquisition scan for the preoperative assessment of cardiac risk. Surg Gynecol Obstet. 1988;167:234-238.
WEB OF SCIENCE
| PUBMED
70. Franco CD, Goldsmith J, Veith FJ, et al. Resting gated pool ejection fraction: a poor predictor of perioperative myocardial infarction in patients undergoing vascular surgery for infrainguinal bypass grafting. J Vasc Surg. 1989;10:656-661.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
71. Kazmers A, Cerqueira MD, Zierler RE. Perioperative and late outcome in patients with left ventricular ejection fraction of 35% or less who require major vascular surgery. J Vasc Surg. 1988;8:307-315.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
72. Davies MJ, Cronin KD, Domaingue CM. Pulmonary artery catheterisation: an assessment of risks and benefits in 220 surgical patients. Anaesth Intens Care. 1982:10:9-14.
73. Berlauk JF, Abrams JH, Gilmour IJ, O'Connor SR, Knighton DR, Cerra FB. Preoperative optimization of cardiovascular hemodynamics improves outcome in peripheral vascular surgery: a prospective randomized trial. Ann Surg. 1991;214:289-299.
WEB OF SCIENCE
| PUBMED
74. Whittemore AD, Clowes AW, Hechtman HB, Mannick JA. Aortic aneurysm repair: reduced operative mortality associated with maintenance of optimal cardiac performance. Ann Surg. 1980;192:414-421.
WEB OF SCIENCE
| PUBMED
75. Perloff JF. Clinical recognition of aortic stenosis: the physical signs and differential diagnosis of the various signs of obstruction to left ventricular outflow. Prog Cardiovasc Dis. 1968;10:323-330.
FULL TEXT
76. Roth RB, Palacios IF, Block PC. Percutaneous aortic balloon valvuloplasty: its role in the management of patients with aortic stenosis requiring major noncardiac surgery. J Am Coll Cardiol. 1989;13:1039-1041.
ABSTRACT
77. Levine MJ, Berman AD, Safian RD, Diver DJ, McKay RG. Palliation of valvular aortic stenosis by balloon valvuloplasty as preoperative preparation for noncardiac surgery. Am J Cardiol. 1988;62:1309-1310.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
78. O'Keefe JH, Shub C, Rettke SR. Risk of noncardiac surgical procedures in patients with aortic stenosis. Mayo Clin Proc. 1989;64:400-405.
WEB OF SCIENCE
| PUBMED
79. Hammermeister KE, Henderson WG, Burchfiel CM, et al. Comparison of outcome after valve replacement with a bioprosthesis versus a mechanical prosthesis: initial 5 year results in a randomized trial. J Am Coll Cardiol. 1987;10:719-732.
ABSTRACT
80. Edmunds LH. Thromboembolic complications of current cardiac valvular prostheses. Ann Thorac Surg. 1982;34:96-106.
FREE FULL TEXT
81. The Stroke Prevention in Atrial Fibrillation Investigators. Predictors of thromboembolism in atrial fibrillation, I: clinical features of patients at risk. Intern Med. 1992;116:1-5.
82. Gainey SP, Robertson DM, Fay W, Ilstrup D. Ocular surgery on patients receiving long-term warfarin therapy. Am J Ophthalmol. 1989;108:142-146.
WEB OF SCIENCE
| PUBMED
83. Ramstrom G, Sindet-Pedersen S, Hall G, Blomback M, Alander U. Prevention of postsurgical bleeding in oral surgery using tranexamic acid without dose modification of oral anticoagulants. J Oral Maxillofac Surg. 1993;51:1211-1216.
WEB OF SCIENCE
| PUBMED
84. White RH, McKittrick T, Hutchinson R, Twitchell J. Temporary discontinuation of warfarin therapy: changes in the international normalized ratio. Ann Intern Med. 1995;122:40-42.
FREE FULL TEXT
85. Stein PD, Alpert JS, Copeland J, Dalen JE, Goldman S, Turpie AGG. Antithrombotic therapy in patients with mechanical and biological prosthetic heart valves. Chest. 1992;102(suppl):445S-455S.
86. Travis S, Wray R, Harrison K. Perioperative anticoagulant control. Br J Surg. 1989;76:1107-1108.
WEB OF SCIENCE
| PUBMED
87. Tinker JH, Tarhan S. Discontinuing anticoagulant therapy in surgical patients with cardiac valve prostheses: observations in 180 operations. JAMA. 1978;239:738-739.
FREE FULL TEXT
88. Katholi RE, Nolan SP, McGuire LB. The management of anticoagulation during noncardiac operations in patients with prosthetic heart valves: a prospective study. Am Heart J. 1978;96:163-165.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
89. Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis: recommendations by the American Heart Association. JAMA. 1997;277:1794-1801.
FREE FULL TEXT
90. Brown FH. Prevention of bacterial endocarditis. In: Goldman DR, Brown FH, Guarnieri DM, eds. Perioperative Medicine. New York, NY: McGraw-Hill Book Co; 1994:421-434.
91. O'Kelly B, Browner WS, Massie B, Tubau J, Ngo L, Mangano DT. Ventricular arrhythmias in patients undergoing noncardiac surgery. JAMA. 1992;268:217-221.
FREE FULL TEXT
92. Vanik PE, Davis HS. Cardiac arrhythmias during halothane anesthesia. Anesth Analg. 1968;47:299-307.
FREE FULL TEXT
93. Committee on Pacemaker Implantation. Guidelines for implantation of cardiac pacemakers and antiarrhythmia devices: a report of the American College of Cardiology/American Heart Association Task Force on Assessement of Diagnostic and Therapeutic Cardiovascular Procedures (Committee on Pacemaker Implantation). Circulation. 1991;84:455-467.
FREE FULL TEXT
94. Berliner D, Okun M, Peters RW, Carliner NH, Plotnick GD, Fisher ML. Transcutaneous temporary pacing in the operating room. JAMA. 1985;254:84-86.
FREE FULL TEXT
95. Bellocci F, Santarelli P, DiGennaro M, Ansalone G, Fenici R. The risk of cardiac complications in surgical patients with bifascicular block: a clinical and electrophysiologic study in 98 patients. Chest. 1980;77:343-348.
FREE FULL TEXT
96. Erdman S, Levinsky L, Servadio C, Stoupel E, Levy MJ. Safety precautions in the management of patients with pacemakers when electrocautery operations are performed. Surg Gynecol Obstet. 1988;167:311-314.
WEB OF SCIENCE
| PUBMED
97. Shapiro WA, Roizen MF, Singleton MA, Morady F, Bainton CR, Gaynor RL. Intraoperative pacemaker complications. Anesthesiology. 1985;63:319-322.
FULL TEXT
|
WEB OF SCIENCE
| PUBMED
98. Simon A. Perioperative management of the pacemaker patient. Anesthesiology. 1977;46:127-131.
WEB OF SCIENCE
| PUBMED
99. American College of Physicians. Guidelines for assessing and managing the perioperative risk from coronary artery disease associated with major noncardiac surgery. Ann Intern Med. 1997;127:309-312.
FREE FULL TEXT
100. Palda VA, Detsky AS. Perioperative assessment and management risk from coronary artery disease. Ann Intern Med. 1997;127:313-328.
FREE FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery: The Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery of the European Society of Cardiology (ESC) and endorsed by the European Society of Anaesthesiology (ESA)
Authors/Task Force Members et al.
Eur Heart J 2009;30:2769-2812.
FULL TEXT
Minimizing perioperative adverse events in the elderly{dagger}
Jin and Chung
Br J Anaesth 2001;87:608-624.
ABSTRACT
| FULL TEXT
|