The Pharmacology and Signaling of Bitter, Sweet, and Umami Taste Sensing
Abstract
Over the last decade, many of the molecular components that mediate the transduction of taste signaling have been elucidated. The chemosensory receptors for taste have been identified as G protein–coupled receptors (GPCRs) and ion channels that are expressed on the surface of highly specialized taste sensory cells. Tastant molecules act as agonists, binding to and stabilizing active conformations of receptors, resulting in the initiation of signal transduction cascades. Taste signaling, therefore, should be amenable to the methods of pharmacology. This review focuses on the GPCR-mediated signaling of bitter, sweet, and umami tastes and emphasizes the opportunities for pharmacologic evaluation.
Introduction
By sampling chemicals in our immediate environment with our tongues, our sense of taste helps us gain information to form a picture of the world. As a subject of scientific inquiry, the study of taste traditionally has fallen under the purview of perceptual sensory psychology. The sensory psychology approach to understanding taste unfolded in accordance with the methods of psychophysics originally developed for the study of vision and audition, and remains a vigorous area of research today (1). The focus mainly has been on the subject’s ability to discriminate one stimulus from another and also on the intensity of a specific stimulus from one subject to the next. In other words, how differently or similarly do people perceive the world through oral sensation? In order to understand the sensations of taste, knowledge of the sensory organ, the tongue, also has been necessary. Accordingly, much study focused on the anatomy of the tongue, the overall organization of the sensory apparatus, and defining the physiological-anatomical unit of sensory reception, the taste bud.
A receptor of some form, it was assumed, had to be present in the taste bud to interact with the chemical signal or “tastant.” Early work in the development of non-nutritive sweeteners established structure–activity relationships (SAR) using human taste tests in what essentially amounted to a pharmacologic characterization of a sweet receptor, well before the molecular identity of the receptor had been revealed (2–4). The door was opened for a new wave of discovery in the field of taste research with the identification of a G protein, gustducin, that was specifically expressed in taste cells (5), and later determined to be critical for the perception of sweet, bitter, and the savory flavor of meat (a taste characteristic now referred to as umami) (6). With the discovery that a G protein was an obligatory taste signaling component, earlier speculation that the elusive taste receptors were likely to be G protein–coupled receptors (GPCRs) (2) was greatly strengthened. A flurry of activity from several laboratories led quickly to the identification of GPCRs for sweet (7–11), bitter (12–14), and umami (7, 15–17). Intensive research efforts currently are aimed at further elucidating the coupling of these GPCRs and their respective signal transduction pathways, as well as characterizing the ligands that interact with them. Now, instead of the majority of taste research working to understand the psychological phenomena of the taste perception, a new focus on the control of taste through application of exogenously applied molecules to block or activate the taste receptors or signal transduction pathways has appeared.
Behavioral Assessment Of Taste
This review of the current knowledge of taste receptors and signaling mechanisms will be prefaced with a description of the psychological and behavioral methods in use for evaluating taste. These approaches are aimed at quantification of the sensory event and are amenable to inclusion into a pharmacologic paradigm that relates taste to receptor function.
Human Psychophysics
The three most commonly used methods to assess taste are absolute threshold measures, recognition threshold measures, and supra-threshold measures. Absolute threshold is the lowest concentration of a tastant that can be detected by a subject as some kind of taste (1). Often, the absolute threshold concentration is obtained using a forced-choice discrimination design, where subjects are given two or three samples, one of which contains a concentration of tastant and the other(s) water, and are asked to identify the tastant solution (18). The sample presentation can be randomized (19) or progress in an ascending order of concentrations from subthreshold to higher concentrations (20, 21). Random guessing results in 50% correct choices, and 75% correct choice rate is usually taken as the threshold for detection of a taste (20). Alternatively, a short series of correct responses consecutively occurring on a specified number of trials also is considered to define the detection threshold (21). Similar approaches are used to determine the recognition threshold, which is the lowest concentration that a subject reports as having a specific taste (1). Threshold detection data often yield very steep concentration-effect functions (19, 20) similar to those obtained in drug discrimination experiments, where responses appear to be more quantal than the graded function predicted solely from mass-action receptor occupancy (22, 23). Nevertheless, detection thresholds have been reported to correspond to EC50 values obtained from in vitro taste receptor assays (19, 20). Suprathreshold measures attempt to quantify taste stimulus intensity. In practice, the relativity of taste intensity complicates quantification. The perceived intensity of the taste stimulus can vary substantially across individuals and considerable effort has been applied to development of data normalization techniques (1, 24). Suprathreshold concentration-intensity functions are more graded (i.e., less quantal characteristics) than the results from threshold measures experiments (20). Relating the function directly to receptor activity could be problematic for bitter agonists, however, because multiple receptors and signaling pathways could be brought into play as tastant concentrations rise. For example, transgenic animals deficient of critical components of bitter receptor signaling pathways still avoid high concentrations of the bitter compounds denatonium benzoate and quinine (25, 26). Suprathreshold intensity measurements might not have the acuity to resolve the expected multiple mechanisms that participate in dose–response functions of bitter compounds (20); however, concentration-intensity functions have proven more amenable to studying sweet receptors (3, 27).
Animal Models
Dose-response functions are readily achieved with some excellent taste-directed animal models. Three commonly used models are the two-bottle taste preference, brief access taste assay, and taste discrimination.
In the two-bottle taste preference experiment, animals are given free access to each of two bottles, one containing water and the second an equal volume of tastant solution, in the home cage (6, 28–30). The volume missing from each bottle is measured at the end of the test period (typically between twenty-four to forty-eight hours) and a preference ratio of tastant to water consumed is calculated. Often, the preference ratio is obtained by dividing the volume of tastant solution consumed by the total volume of consumed liquid (i.e., tastant/(water + tastant). A tastant solution is defined as aversive if the resulting preference ratio is <0.5, appetitive if >0.5, and taste-neutral if equal to 0.5 (6, 29). The two-bottle taste preference is the simplest animal model available for obtaining inferences about taste. Preference ratios vary as a function of tastant concentration and therefore can be used to determine oral aversive or appetitive potencies. Results from these experiments must be interpreted cautiously, however, because post-ingestive effects of the tastant solution consumed can impact on the data independent of taste. For example, NaCl solutions presented in an ascending order of concentrations yield concentration-preference ratio functions that are substantially different from those obtained by a descending order in two-bottle assays (31). Furthermore, strain-dependent differences in preferences for sucrose and saccharin evident in a twenty-four hour two-bottle test disappeared when the mice were tested a second time (32). Preference or avoidance based on taste alone cannot be inferred because additional physiological effects that result following ingestion of tastant quantities consumed ad libitum over protracted periods of time could influence intake of the solutions. Another disadvantage of the two-bottle approach is that it can take weeks or longer to acquire enough data for a dose-response function.
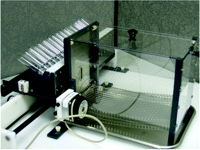
A better system that is faster and avoids much of the potential post-ingestive effect of the two-bottle taste preference approach is the brief access taste assay (33, 34). Animals, often rodents, are mildly water-deprived and thereby motivated to sample from one of multiple spouts that are presented one at a time through a port, usually in random order, in an apparatus called the Davis Rig (35) or “lickometer” (Figure 1⇓). The spouts are attached to bottles arranged in a rack, or “lick block,” that is moved back and forth by a linear actuator directed by a computer program. Each bottle contains only a few milliliters of liquid, either water (or vehicle) or any of several tastant solutions. Animals have only a short period of time to sample a solution before a time-operated shutter closes to prevent further access to the spout. Each time the tongue makes contact with the spout an imperceptible electric current running though the system is grounded, and the event is recorded as a “lick.” Typically, a thirsty mouse will emit thirty to fifty licks within a five-second trial on a spout delivering water, but lick rates are nearly completely suppressed by a highly aversive tastant, such as 100 μM sucrose octaacetate (34) or 1 mM quinine hydrochloride (36, 37). Taste thereby becomes operationally defined as a departure from the water lick rate. By presenting a range of tastant concentrations, a full dose-response function of lick rate can be obtained over a thirty-minute time period (33, 34, 36). The dose-response functions for aversive compounds such as quinine are robust and stable from day to day (36). The dose-response functions for appetitive solutions such as sucrose are more difficult to obtain from a thirsty animal because the lick rate for water can be near maximal. Full dose-response functions still can be obtained by normalizing all data to each individual animal’s maximum potential lick rate per trial (36, 38). Alternatively, animals not water deprived have been used to generate dose-response functions for sucrose (39).
Davis Rig “Lickometer” for the brief-access taste assay. Rodents that are mildly water-deprived lick solutions from up to sixteen bottles arranged in the “lick block.” Spouts from each bottle are presented one-at-a time through the port in the front of the cage. A “lick” is recorded each time the rodent’s tongue makes contact with the spout.
A more direct assessment of taste can be obtained from operant taste discrimination, a paradigm that is essentially the equivalent of drug discrimination in behavioral pharmacology. A standard tastant solution is trained as a discriminative stimulus for food or water reinforcement of an operant task, such as pressing a lever (40–42) or licking a water spout (43–45). The simplest taste discrimination is set up so that the standard taste cue (for example, 100 mM sucrose) is repeatedly associated with reinforcement exclusively on one of two available manipulanda (any device that the test animal must operate to obtain a reinforcer), and the second manipulandum is associated with a taste-neutral cue (water). Once the discrimination is trained, differential responding on the manipulanda indicates the similarity or disparity of taste cues in novel test solutions to those of the standard tastant. All taste modalities have been evaluated by utilizing this paradigm. Rodents are most often used as subjects in operant taste discrimination (40, 41, 43–45), but methods also have been developed for primates (40, 42, 46). Analogous to the drug discrimination paradigm of behavioral pharmacology, taste discrimination has been used to demonstrate a rightward shift in the NaCl taste discrimination dose-response curve by amiloride, a competitive antagonist of the epithelial sodium channel (45). Thus, taste discrimination likely will prove valuable in pharmacologic evaluation of taste responses as high affinity, selective molecular tools become available.
Receptors And Signaling Pathways
The first taste receptor candidates cloned from taste cells, originally designated TR1 and TR2, were identified as class C GPCRs (7). Later, a related GPCR taste receptor with similar structural characteristics was discovered (8–11). Together they became known as the T1R family of receptors for sweet (8–11) and umami (15–17) tastes. Shortly after the discovery of the genes for T1R1 and T1R2, a second larger family of putative taste GPCR genes (T2R) was identified and anticipated to encode bitter receptors (12, 13). The murine T2R5 (mT2R5) receptor was the first taste GPCR to be unequivocally associated with a tastant ligand (14).
Bitter Receptors: The T2R Family of G Protein–Coupled Receptors (GPCRs)
Mouse strains had long been recognized that varied in sensitivity to specific bitter substances, such as cyclohexamide (47) and sucrose octaacetate (48). The genetic variation underlying the different sensitivities mapped to the distal end of chromosome 6 (48), and a cluster of twenty-five genes encoding candidate bitter receptors was identified later (12, 13). Several of these candidates were expressed with promiscuous G proteins in null cell lines lacking any taste receptors and screened for their responses to a panel of twelve bitter agents in a calcium signaling assay. Cells expressing the mT2R5 responded robustly and exclusively to cyclohexamide with a potency of 0.5 μM, which was essentially the same potency of cyclohexamide in a mouse brief access taste aversion assay (14, 49). Finally, disruption of the gene for mT2R5 in mice rendered them insensitive to cyclohexamide in brief-access taste aversion (49). These results provided solid evidence to support the deorphaning of a bitter taste receptor.
More genetic evidence for T2R bitter receptors has been provided by the variation humans display in ability to sense the intensely bitter taste of 6-n-propyl-2-thiouracil (PROP) and phenylthiocarbamide (PTC) (50). Taste-test subjects have been designated as “non-tasters,” “tasters,” and “supertasters” according to the intensity of their responses to these substances (51). The variation in taste sensitivites follows expected Mendelian genetics, and the trait has been mapped to chromosomes 5 and 7 (52). Subsequent research using positional cloning identified a single GPCR-encoding gene that was a likely candidate for mediating the taste of PROP and PTC (53). The receptor, T2R38, was expressed in recombinant cells that subsequently responded in a calcium signaling assay to both PROP and PTC with potencies similar to those reported in human taste tests (20). Mice normally do not respond to low concentrations of PTC, but transgenic mice that express the human T2R38 display marked sensitivity to both PTC and PROP (49). Furthermore, the variation in human threshold sensitivity to the taste of PTC has been linked to mutations in the gene for T2R38 resulting in polymorphisms of the receptor at residues 49 (where either proline or alanine is encoded), 262 (alanine or valine), and 296 (valine or isoleucine) (53). Recombinant T2R38 receptors containing the equivalent mutations responded to PTC and PROP in calcium signaling assays with EC50s that closely corresponded to threshold values obtained in psychophysical tests from individual subjects of each haplotype (20).
In a calcium signaling assay, human T2R16 (hT2R16) was identified as a receptor that mediates the bitter taste of the glucopyranosoide salicin, with an EC50 of 70 μM (19). SAR evaluation revealed stereoselectivity to the receptor activation, with a critical requirement for the beta conformation of the D-glucopyranoside moiety. Several other phenyl-β-d-glucopyranosides showed activity, but apparent affinity decreased approximately 200-fold when the phenyl group was replaced by a methyl moiety. A panel of nine other structurally unrelated bitter tastants failed to evoke responses from the hT2R16-expressing cells. The SAR and potencies measured in the in vitro assay closely matched results from a human bitterness recognition threshold test, as did the selectivity of desensitization of responses in both in vitro and human tests (19).
Bitter agonists now have been reported to be associated with several other mouse, rat, and human T2Rs (Table 1⇓). Activation of most of the receptors appears to be quite selective, if not specific, for their respective cognate ligands. For example, mT2R5, hT2R43, and hT2R47 show specificity for single ligands (cyclohexamide, aristolochic acid, and denatonium, respectively) out of seventeen bitters tested (54). The orthologs (that is, genes from different species that possess the same functional activity) hT2R4 and mT2R8 responded to high concentrations of the structurally disparate compounds PROP and denatonium, but those two compounds were the only active agonists out of a battery of fifty-five compounds tested (14). So far, the exceptions to the high selectivity exhibited by T2Rs appear to be hT2R7 (54) and hT2R14 (55), both of which have been reported as “broadly tuned” (i.e., less specific) in that they respond to relatively high concentrations of several unrelated bitter ligands. However, a general property of agonist promiscuity for hT2R14 apparent from a cell-based calcium signaling assay (55) was not corroborated by results obtained in a membrane-based [35S]–GTPγS binding assay using a different set of seventeen bitter ligands, where hT2R14 was responsive only to aristolochic acid (54).
Agonists for Cloned T2R Bitter Receptors: In Vitro Determinations
Multiple “broadly tuned” receptors might have evolved to protect organisms from ingesting any of an enormous set of potentially toxic compounds (55–57). Such a teleonomic explanation would be consistent with the discovery of a large family of approximately twenty-five to thirty receptors expressed in human and mouse tongue (12–14) that are candidate bitter receptors. However, intensive investigation of the T2Rs has yielded little evidence that these taste receptors are promiscuous with respect to their agonist ligands. Even the declaration of Berhens et al. (55) that the eight identified T2R14 agonists have little in common is debatable (Table 1⇑). One easily could argue that the eight listed T2R14 agonists represent only three distinct structural categories. Thus, at this point, it is not clear that T2R receptors account for all of the aversive taste sensation from an almost limitless supply of bitter ligands. But if not T2Rs, what else could mediate bitterness?
One proposal not warmly embraced by the taste-science community is that general structural characteristics of amphipathicity and amphiphilicity enable compounds to rapidly insert into cell membranes where they could directly activate G proteins or other signaling molecules independent of receptor occupancy (58–60). In this view, the bitter sensations imparted by high concentrations of compounds bearing these properties (for example, most pharmaceuticals) would have to be a default response resulting from indiscriminant activation of most, if not all, G proteins in the tongue, because, presumably, sweet and umami taste pathways also would be activated. Perhaps this unpopular hypothesis should be reconsidered; it seems to offer a parsimonious explanation for the bitterness of the vast numbers of otherwise unrelated chemical classes.
Sweet and Umami Receptors: The T1R GPCRs
The T1R1, T1R2, and T1R3 receptors are class C GPCRs, characterized by large N termini, that heterodimerize to form functional taste receptors for sweet or umami sensing. The general structural features of T1Rs and their tendency to form heterodimers are shared with other members of the class C receptors, such as the γ-amino butyric acid type B (GABAB) and metabotropic glutamate receptors (61).
T1R3 was discovered by mapping a portion of the human genome that is syntenic to the mouse Sac locus, a chromosomal region previously recognized as the chief genetic determinant of strain differences in sensitivity to sucrose and saccharin (48, 62, 63). T1R3 was identified as the only GPCR within the Sac locus (8–10, 64) and, therefore, a likely candidate for the sweet receptor. The expression pattern of T1R3 overlapped with those of the previously discovered T1R1 and T1R2 receptors, each of which were known to be selectively expressed in distinct subsets of taste cells (7). The co-localization of T1R3 with the other T1Rs suggested that heterodimerization might be necessary to form functional taste receptors (8, 11). Indeed, when rat (11) or human (16) T1R2 and T1R3 were coexpressed with promiscuous G proteins in HEK293 cells, robust responses to sweet tastants were observed in a calcium signaling assay. Similarly, coexpression of T1R1 and T1R3 generated recombinant cells that responded exclusively to umami l-amino acids, such as glutamate (15, 16). Expressed singly, the T1Rs respond weakly, if at all, to tastant stimuli in vitro (11). The physiologic roles of these receptor heterodimers was established in studies using transgenic mice. Knockout mice devoid of T1R1, T1R2, or T1R3 were generated and their sensitivities to sweet and umami tastants mirrored the results obtained in the recombinant cell based assays; that is, single T1R1- or T1R2-knockout mice were insensitive to umami or sweet tastants, respectively, whereas T1R3-knockouts were unable to taste both sweet and umami in brief-access taste assays (17). Thus, the sweet receptor is a T1R2-T1R3 heterodimer and the umami receptor is a T1R1-T1R3 heterodimer.
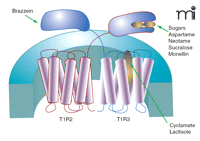
Some detail of ligand-receptor interaction has been worked out for the sweet receptor. Much about the ligand-binding selectivities of the sweet receptor were anticipated from the cross-species differences between rodents and humans to a variety of non-nutritive sweetener and sweet proteins (65, 66). For example, humans can detect the sweet proteins monellin and thaumatin, as well as aspartame at micromolar concentrations whereas rodents do not respond to concentrations of these in the millimolar range (65–67). Noting that the amino acid sequences of mouse and human T1Rs generally differ by approximately 30%, Zhao et al. (17) engineered transgenic mice to express the human form of T1R2 in place of native murine T1R2, specifically in taste cells. These humanized sweet receptor mice gained sensitivity to aspartame, glycyrrhizic acid, thaumatin, and monellin, but still did not respond to the human sweet ligand neohesperidin dihydrochalcone (17). These data suggested that the principal binding sites for thaumatin, monellin, and aspartame are on the T1R2 monomer. Using a similar cross-species chimera approach in cell-based calcium signaling assays, Jiang et al. (68) demonstrated that the binding site of another sweet protein, brazzein, was located on the hT1R3 subunit. By systematically replacing sequences of the mouse T1R3 with hT1R3 sequence, a cysteine-rich region of the N-terminal domain between residues 536 and 545 of hT1R3 was determined critical for brazzein binding. Domain swapping further revealed that the binding site of monellin was localized to the N terminus of human T1R2 (68). The use of rat–human sweet receptor chimeras has shown that aspartame and neotame also bind to the hT1R2 N-terminal domain (69). In contrast, the non-nutritive sweetener cyclamate and the sweet taste inhibitor lactisole bind distal to the N-terminal domain of T1R3, enabling these ligands to affect the functioning of both sweet and umami receptor activity (69, 70). Hypothesizing that the N termini of the sweet receptor subunits were the likely binding site of sugars, Nie et al. (71, 72) developed a spectroscopic assay for obtaining ligand affinities for sucrose, glucose, and the non-nutritive sweetener sucralose. Intrinsic fluorescence and circular dichroism spectra of solubilized N termini from mouse T1R2 and T1R3 changed as a function of ligand concentration. The resulting binding curves yielded KD values for the three sweet ligands, revealing stronger affinity of sucrose and glucose to T1R2 than to T1R3, whereas the converse was true for sucralose (71). Figure 2⇓ summarizes the current understanding of ligand binding sites for the T1R2-T1R3 sweet receptor.
The human T1R2-T1R3 sweet receptor. The functional receptor is a heterodimer composed of a T1R2 and a T1R3 class C GPCR. Binding for most sweet ligands occurs on the T1R2 unit. Sugars may interact with the large N termini of both T1R2 and T1R3, whereas the sweet protein brazzein binds to a cysteine-rich region of the T1R3 N terminus. Cyclamate and the sweet-taste inhibitor lactisole bind to the transmembrane-spanning domains of T1R3.
Gustducin
The first component of the taste signaling pathway was identified as a G protein and named gustducin because of its highly selective expression in approximately 25–30% of taste cells (5, 6). Gustducin is a member of the Gi family of G proteins and is most closely related to the transducins, sharing approximately 80% amino acid sequence identity (73, 74). The role of gustducin in taste was confirmed by behavioral experiments using transgenic mice deficient of gustducin (6). In a taste preference test, gustducin knockout mice were indifferent to the aversive taste of quinine and other bitters as well as to the taste of sucrose, but were sensitive to salty and sour solutions. However, more recent evidence in brief-access taste assays has suggested that gustducin’s role in the signal transduction of sweet and umami is perhaps less important than previously thought (38).
Second Messenger Systems
The final thirty-eight amino acids in the C terminus of the α subunits of gustducin and the transducins are identical (5, 73, 74), and this region is associated with G protein–effector coupling. Early work from Margolskee suggested that, like its close relative transducin, gustducin interacts with phosphodiesterases (73, 75).
Several studies have implicated adenosine 3′,5′-monophosphate (cAMP) as a second messenger in taste signaling (76–82). For example, the calcium-sensitive adenylyl cyclase A8 is expressed in rat taste buds (79) and is thought to mediate the tastant-evoked rise in cAMP in taste cells (81). Furthermore, a taste cell–specific cyclic nucleotide–gated ion channel has been found expressed near the pore of the taste bud in rats (82). Although evidence suggests a role for the adenylyl cyclase pathway in taste signaling, more studies will be required before the function of cAMP in taste is elucidated.
In contrast, the relationship of the inositol phosphate signaling cascade to taste is clearer. Both phospholipase C–β2 (PLCβ2) and the inositol phosphate type III receptor (IP3R3) are locally coexpressed in taste cells (83, 84). Applying bitter, sweet, or umami tastants to lingual tissue or isolated taste cells results in increased intracellular IP3 and calcium responses (85–91). The critical role of PLCβ2 in taste signaling is greatly strengthened by studies in transgenic mice: PLCβ2 knockout mice are insensitive to the aversive taste-effects of cyclohexamide and to the appetitive taste-effects of sucrose and umami (92). Curiously, no report has yet shown that PLC inhibitors, such as U73122, can inhibit taste transduction.
Transient Receptor Potential–Melastatin 5 (TRPM5)
The non-selective cation channel transient receptor potential–melastatin 5 (TRPM5) is a downstream signaling protein that plays an obligatory role in propagation of signals initiated by sweet, bitter, or umami tastants (25, 92, 93). TRPM5 is a member of the superfamily of related TRP ion channels, many of which are involved in a variety of sensory functions (94). The structure of a single monomer of TRPM5 is composed of a relatively short N terminus, six transmembrane-spanning domains, and a long C terminus that might allow multimerization with other TRP proteins to form a functional unit (95, 96). Evidence from studies of other TRP channels suggests that the functional unit is made up of four individual TRP proteins (94). Although some TRP channels are capable of heteromultimerizing (97, 98), to date no evidence exists indicating that TRPM5 associates with other TRP channel family proteins to form a functional channel (94).
The expression pattern of TRPM5 is highly selective, with expression in lingual tissue localized to type II taste cells (discussed below) where it coexists with T1Rs and T2Rs, gustducin, and PLCβ2 (93, 99, 100). Additional expression of TRPM5 has recently been detected in specialized cells of the gastrointestinal tract (101, 102); pancreas and prostate (101); and olfactory neurons (103). The presence of TRPM5 with other taste-signaling proteins in the gastrointestinal tract suggests some nutrient-sensing involvement in digestive functions (102).
An obligatory role for TRPM5 in taste has been established using transgenic animals. Mice deficient of TRPM5 exhibit a taste phenotype essentially identical to that of PLCβ2 knockout mice, with pronounced and specific deficits in the ability to detect sweet, bitter, and umami tastants (25, 92), strongly suggesting that the two signaling proteins act in series.
The conductance of TRPM5 is gated by intracellular calcium and can be activated by calcium release from intracellular stores. Thus, a TRPM5-dependent current can be elicited by stimulation of a PLC-linked GPCR (92, 93, 104). Desensitization of the TRPM5 conductance occurs rapidly, with an inactivation–deactivation time constant of approximately three to six seconds (95, 96), and is thought to be partly hastened by PLC-mediated loss of inositol lipids from the plasma membrane (104). In addition to regulation by intracellular calcium and local phospholipids, TRPM5 activity is sensitive to temperature. Recent studies (105) have shown that sodium conductance of TRPM5 is enhanced by elevated temperature (ranging from 15–35 °C), a range that can occur on or near the tongue’s surface with contact of hot or cold foods and beverages. The temperature sensitivity of TRPM5 appears to translate into a temperature-dependent enhancement of signaling in chorda tympani, one of the major innervations involved in taste sensation. Temperature modulation of TRPM5 could therefore underlie perceived temperature-dependent changes in the intensity of some taste sensations (106, 107).
Taste Receptor–containing Cells
Taste Buds and Taste Cells
The sensory organelle for taste is the taste bud, a garlic clove-like structure that is contained within papillae on the tongue’s surface. There are three general areas of the tongue where the papillae and taste buds are concentrated: 1) circumvallate papillae; 2) foliate papillae, and 3) fungiform papillae. In humans, each circumvallate papilla, found in the posterior region of the tongue, can contain thousands of taste buds. Taste buds densities range into the hundreds in foliate papillae, which are located on the outer edge near the back of the tongue. Finally, fungiform papillae are found in the anterior third of the tongue and contain only a few taste buds each (56, 108–110). The garlic clove appearance of the taste bud derives from the elongated polarized taste cells that are packed side-by-side within to form a bulbous structure surrounded by epithelial cells. The narrow apical ends of the taste cells are packed tightly together and each extends microvilli into a pore in the surface of the tongue (109–113).
Taste cells are categorized into three types, designated I, II, and III, distinguished unequivocally by specific markers (99, 114) and physiological characteristics (115). A fourth type, basal cells, also exists within the taste bud and is believed to be a progenitor of the other types as needed in the course of cell turnover, which occurs every ten to fifteen days (116).
Little is known about type I cells except that they might have glial characteristics (117) and that they express the nucleoside triphosphate diphosphohydrolase-2 (NTPDase2) (118). Like type II cells, the type I cells possess an apical microvillus structure. Recently, the receptor for sour taste, a heterodimer of the TRP-related polycystic kidney disease (PKD) 1L3 and 2L1 ion channels, has been identified. These channels are expressed in taste cells that are not type II (119–121). No evidence has linked sour receptors to the type I cell, although it is tempting to speculate that type I cells might be sour-sensing.
Type II cells are the taste receptor cells for bitter, sweet, and umami (13, 89, 92) and are identified by their expression of PLCβ2, TRPM5, and IP3R3 (99, 114). Subsets of type II cells also express gustducin and different types of taste receptors (5, 8, 13, 15). Voltage-dependent calcium currents are not present in type II taste cells, but small voltage-dependent sodium and potassium currents have been measured (100, 115).
In contrast, Type III cells are distinguished from type II cells by the presence of voltage-gated calcium channels (99, 115, 122), SNAP-25 (synaptosome-associated protein of 25,000 daltons) (99, 123)—a component of the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex that mediates vesicular fusion to the membrane during exocytosis (124)—and the synaptic extracellular neural cell adhesion molecule (NCAM) (99, 114). These characteristics suggest that type III cells, but not type II cells, are capable of forming synapses with sensory neurons. Although all of the receptors and signal transduction machinery exists in the type II cells, the capacity for synapse formation with sensory neurons appears to be relegated solely to the type III cells. These observations have led to the conclusion that intercellular communication must occur between the taste-sensing type II cells and the synapse-capable type III cells (125).
Cell-to-Cell Communication
Strong cases have been made for both ATP and serotonin (5-HT) as two important humoral factors that could mediate intercellular signaling within the taste bud (118, 126–133). For instance, tastant-stimulated release of ATP from lingual tissue preparations has been demonstrated (128), and mice lacking the ionotropic purinergic P2X2 and P2X3 receptors exhibit dramatic taste deficits similar to those reported for PLCβ2- and for TRPM5-knockout mice (128).
Serotonin (5-HT) (129, 130), 5-HT transporters (130), and several 5-HT receptors (131) also have been detected in taste buds. Recombinant cells that express high numbers of a particular GPCR can serve as “biosensors” for detecting the presence of that receptor’s ligand. The use of biosensors has proven a powerful technique for demonstrating the tastant-stimulated release of 5-HT and ATP from taste buds and taste cells. In calcium imaging experiments, fura-2-loaded CHO cells expressing 5-HT2C receptors were used as biosensors to detect the release of 5-HT from isolated taste buds (132, 133). A single biosensor cell held at the end of a micropipette was unresponsive to a variety of tastants or 50 mM KCl perfused into the extracellular medium but responded with robust calcium signals when treated with 5-HT. However, when in close apposition to an isolated taste bud, strong calcium responses were recorded from the biosensor cell in the presence of either tastant or KCl. These responses were reversibly blocked by the 5-HT receptor antagonist mianserin, indicating that the factor secreted by the tastant-stimulated taste buds was 5-HT (132).
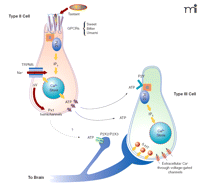
As mentioned above, only type II cells respond to bitter, sweet, and umami tastant stimulation, whereas type III cells, by virtue of their voltage-gated calcium channels, can be depolarized by raising the extracellular concentrations of KCl. These characteristics of taste cells, coupled with the use of biosensors, have been exploited to elucidate the mechanisms of intercellular communication between the two cell types (122, 133). In elegant experiments biosensor cells that express P2Y (122) or P2X2/3 (133) purinergic receptors were also used to demonstrate that ATP was secreted exclusively by type II cells (122, 133). Stimulated ATP secretion was not exocytotic, but occurred through pannexin-1 hemichannels actuated by the combined effects of intracellular calcium and a membrane depolarization likely to be triggered by TRPM5 activity (122, 133). ATP did activate purinergic receptors on the surface of type III cells and, consequently, stimulated the type III cells to release 5-HT by exocytosis (133). The precise nature of the interaction between type III cells and sensory neurons has yet to be defined. A schematic representation of type II and type III taste cell signaling is shown in Figure 3⇓.
The taste cell signaling model. Tastant molecules, acting as agonist ligands, bind to and stabilize the active conformation of GPCRs in the apical microvilli of type II taste cells. The active receptor interacts with gustducin and other G proteins that consequently release their βγ subunits to stimulate PLCβ2. Hydrolysis of membrane PIP2 by PLCβ2 generates inositol trisphosphate (IP3) that, in turn, interacts with and opens the IP3R3 channel on internal calcium stores. The increase in [Ca2+]i activates TRPM5-mediated changes in membrane potential (ΔV), and subsequently ATP is released through the pannexin-1 hemichannel. Secreted ATP acts on P2Y purinergic receptors on type III taste cells, causing the exocytotic release of 5-HT into the synapse between type III cells and afferent sensory neurons. ATP from type II cells is also believed to stimulate afferent sensory neurons. Model diagrammed according to (128, 133) and Stephen Roper, personal communications.
Conclusion
Pharmacology arguably could be defined as the study of the precise control of biological functions through exogenous application of chemicals. Over the last century, principles of pharmacology have been developed to provide methodological tools that shed light on receptor-ligand interactions and how they relate to physiology. We now have the potential to control precisely the perception of taste by controlling the peripheral sensory apparatus and its function directly with small molecules. In fact this is what humans have always done in an imprecise way by adjusting the flavors of foods. Each flavoring ingredient now can be regarded as an agonist, or perhaps, an allosteric modulator. With the ability to precisely control taste sensation at the level of the sensory receptors in the tongue, we might be able to modulate, turn on or off, or fine tune taste sensations at will.
Food industries will find taste modulators valuable, particularly if allosteric modulators become available that allow concentrations of some nutritive tastants, like sucrose and salt, to be reduced without loss of any of the desirable taste qualities they provide. On the other hand, the pharmaceutical industry needs blockers of aversive tastes to help improve patient compliance with regimens of unpalatable orally administered therapeutics. With these issues as driving forces, many food and pharmaceutical companies now are beginning to turn their attentions and resources to the pharmacologic intervention of taste. The fact that the sensory receptors are GPCRs or ion channels should immediately be recognized by pharmacologists as targets amenable to the techniques and instrumentation for high-throughput screening (HTS) and SAR-based chemistries to identify novel modulators of taste. The field of taste is rife with new opportunities to apply the principles of pharmacology.
- © American Society for Pharmacology and Experimental Theraputics 2007
References

R. Kyle Palmer, PhD, is Associate Director of HTS and Pharmacology at Redpoint Bio Corp, a biotechnology firm that identifies and develops compounds to improve the taste of pharmaceutical, food and beverage products. Address correspondence to kpalmer{at}red-pointbio.com; fax (609) 860-5900.