|
 
 |
|
ORIGINAL ARTICLE |
|
|
|
| Year : 2012 | Volume
: 18
| Issue : 2 | Page : 222-225 |
| |
Lack of association between the G-660C polymorphism in the dopamine transporter gene (SLC6A3) and schizophrenia in the Iranian population
Ali M Foroughmand, Hamid Galehdari, Bentalhoda Tirband Dastgerdi, Saeed Reza Khatami, Maryam Haidari
Department of Genetics, Faculty of Sciences, Shahid Chamran University, Ahwaz, Iran
| Date of Web Publication | 8-Sep-2012 |
Correspondence Address:
Ali M Foroughmand
Department of Genetics, Faculty of Sciences, Shahid Chamran University, Ahwaz
Iran
 Source of Support: None, Conflict of Interest: None
DOI: 10.4103/0971-6866.100773

 Abstract Abstract | | |
Background: Dopaminergenic system plays an essential role in the plasticity of the human brain. The dopamine transporter gene (SLC6A3) mediates active reuptake of dopamine from synapsis, terminates dopamine signals, and therefore, is implicated in a number of dopamine-related disorders like psychosis. Variations in the form of single nucleotide polymorphisms in the core promoter of the SLC6A3 gene are reported to be involved in the pathogenesis of schizophrenia. In this study, we also attempted to establish the possible role of the polymorphism G-660C in the SLC6A3 gene promoter in schizophrenia in a case-control study.
Materials and Methods: The allele and genotype frequency were analyzed in an Iranian cohort of 200 unrelated patients and 200 controls using polymerase chain reaction and restriction fragment length polymorphism.
Results: The genotype frequency for case and control groups was GG 100%, GC 0%, CC 0%, and GG 100%, GC 0%, CC 0%, respectively. The C allele was failed in both groups.
Conclusion: Our data suggest clearly that there is no association between the -660G/C polymorphism and outcome of schizophrenia in the Iranian population.
Keywords: Schizophrenia, dopamine transporter gene, polymorphism, Iranian population, association study
How to cite this article:
Foroughmand AM, Galehdari H, Dastgerdi BT, Khatami SR, Haidari M. Lack of association between the G-660C polymorphism in the dopamine transporter gene (SLC6A3) and schizophrenia in the Iranian population. Indian J Hum Genet 2012;18:222-5 |
How to cite this URL:
Foroughmand AM, Galehdari H, Dastgerdi BT, Khatami SR, Haidari M. Lack of association between the G-660C polymorphism in the dopamine transporter gene (SLC6A3) and schizophrenia in the Iranian population. Indian J Hum Genet [serial online] 2012 [cited 2016 Jun 1];18:222-5. Available from: http://www.ijhg.com/text.asp?2012/18/2/222/100773 |
 Introduction Introduction | |  |
Dopamine is the major neurotransmitter of the neuronal network in the human brain and is involved in the control of motor function, cognition, and behavior. [1],[2] The human dopamine transporter (SLC6A3) is a member of the sodium and chloride-dependent neurotransmitter transporter family and contains 12 transmembrane domains. [3],[4],[5],[6],[7],[8] The dopamine transporter terminates the synaptic transmission by the rapid and specific reuptake of dopamine into the presynaptic nerve terminals. [1],[9],[10],[11],[12] Psychostimulants, such as cocaine and amphetamine, are known to bind the dopamine transporter and inhibit dopamine reuptake. [13] In DAT knockout mice, dopamine clearance is prolonged and the mice show spontaneous hyperactivity. [14],[15],[16],[17],[18] The reduction in dopamine reuptake results in adaptive changes such as a decreased content of dopamine in presynaptic terminals and significant downregulation of D1 and D2 receptor expression. [6]
The dopamine hypothesis of psychosis is a model attributing symptoms of schizophrenia to a disturbed and hyperactive dopaminergic signal transduction. [7],[8] However, several reports suggested strong association between SLC6A3 gene variants and schizophrenia. [6],[9] It has further been shown that the most variants in this gene are located within the putative binding site for transcription factors. The allelic variants would also impact the nature of these response elements. [19],[20],[21],[22] To date, some single nucleotide polymorphisms have been identified in the core promoter of the SLC6A3 gene, which appear to be linked with the outcome of schizophrenia. One of these variants is the T-67A polymorphism, and has previously been investigated by Khodayari et al. in a set of Iranian subjects and controls. Their findings provided tentative evidence for the contribution of SlC6A3 gene core promoter polymorphism to the etiology of schizophrenia in the Iranian population. [20] We attempted also to investigate in a case-control study, the relevance of the G-660C polymorphism, in the Iranian population.
 Materials and Methods Materials and Methods | |  |
Subject
For the case-control study, 200 unrelated patients with a mean age of 43.34 (SD=11.353) were recruited from hospitals in south and south-west of Iran. Of these, 117 were men (63 from south and 54 from south-west) and 83 were women (48 from south and 35 from south-west). The diagnosis of schizophrenia was based on DSM IV criteria. The control group consisted of 200 healthy blood donors with a mean age of 39.43 (SD=11.103), which were matched for gender and ethnicity to the patient cohort. An informed consent was obtained from all participants.
Genotyping
Genomic DNA was extracted from whole blood using the standard salting out method. The G-660C polymorphism in the SLC6A3 gene was screened using the PCR-RFLP method. Sequence of primers used to amplify 260 nucleotides from 5΄-flanking region of SLC6A3, contains -660G/C polymorphism were F: 5΄-TGGAGCCGGATACCAACC-3΄, and R: 5΄-TCCACCTCTGACCAGC-3΄. Following a melting step at 94°C for 5 minutes, 35 cycles of 94°C for 30 seconds, 60°C for 30 seconds and 72°C for 30 seconds was used for amplification and a final elongation step of 72°C for 5 minutes. PCR products were digested overnight at 37°C with 10 units of restriction endonuclease HhaI (Biolabs), which cuts only the G allele, and were analyzed by gel electrophoresis.
Statistical analysis
The Chi-square test was performed to compare genotypes within and between genders. Chi-square tests were calculated using the SPSS 13 statistical package.
 Results Results | |  |
The study of socio-demographic features of the cohort identified that subjects in the case group were significantly lower than those in the control group in terms of marital status and educational level (P<0.05). Control and patient groups were initially classified only by gender and were not statistically different in terms of their socio-demographic features (P>0.05) [Table 1]. The genotypic frequencies of individuals from the 2 geographical regions (south and south-west Iran) were GG 100%, GC 0%, CC 0%, respectively [Table 2]. However, no C allele has been seen among the case-control groups.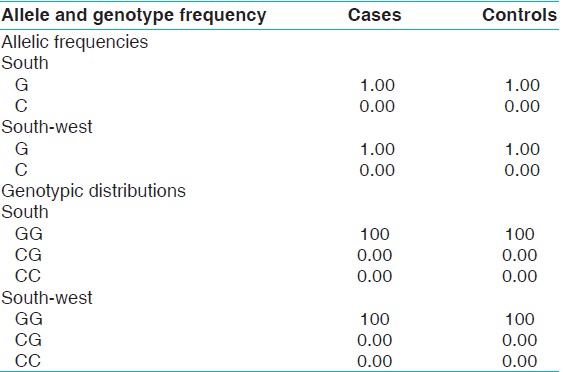 | Table 2: Allelic frequencies and genotypic distribution of the -660G/C polymorphism in the DAT1 gene
Click here to view |
 Discussion Discussion | |  |
The relevance of genetic factors in the etiology of schizophrenia has been demonstrated in many studies, [3] and the hypoexpression of SLC6A3 has been hypothesized as a possible etiology of schizophrenia. [6] Dysregulation of hSLC6A3 expression could be related to a particular contribution of polymorphisms across the gene. [20],[21],[22] The SLC6A3 promoter region displayed 5 polymorphic sites: T-67A, G-660C, C-839T, C-1169G, and T-1476G. They are located within a core region that regulates the activity of luciferase construct with 10-150 times more activity than promoter less construct. Computational analysis of G-660C SNP revealed that this polymorphism is located within a putative Sp1-binding site, and -660C introduces a LF-A1 binding site. Whereas Sp1-binding site are ubiquitously expressed in mammalian cells, the transcription factor LF-A1 interacts with the promoter region of several genes expressed in hepatocytes. [22] Indeed, the functional data about the mentioned SNPs make them attractive to be involved in the etiology of schizophrenia and so far association studies. However, in 2004, Khodayari et al. carried out an association study in a cohort of Iranian subjects and controls. They have found an association between the SLC6A3 gene core promoter polymorphism -67A/T and schizophrenia. [20] In the present survey, the polymorphism -660G/C was proved in a case/control study in a relatively large size of samples. Our results revealed lack of association between this polymorphism and schizophrenia disorder. Studies about European populations showed similar results. [21],[22] But, in contrast to the previous reports, the present study indicates definitely the lack of C allele in 400 individuals of population from south and south-west of Iran. In 800 screened alleles, the G allele was the only one that was observed. The highlight of our results is also the absence of C allele in the gene pool of Iranian population that we studied. Therefore, this polymorphism may not have any role in pathophysiology of schizophrenia, and the -660G/C variant of SLC6A3 gene seems to be not proper candidate for etiology of schizophrenia, at least in Iranian patients.
 Acknowledgment Acknowledgment | |  |
This study was supported in part by grant of Shahid Chamran University of Ahwaz, and we thank from Ahwaz and Shiraz hospitals for collaboration and preparation of samples in this study.
 References References | |  |
| 1. | Hastrup H, Sen N, Javitch JA. The human dopamine transporter forms a tetramer in the plasma membrane: cross-linking of a cysteine in the fourth transmembrane segment is sensitive to cocaine analogs. J Biol Chem 2003;278:45045-8. 
[PUBMED] |
| 2. | Barron AB, Maleszaka R, Vander Meer RK, Robinson GE. Octopamine modulates honey bee dance behavior. Proc Natl Acad Sci U S A 2007;104:1703-7. 
|
| 3. | Vardenbergh DJ, Persico AM, Uhl GR. A human dopamine transporter cDNA predicts reduced glycosylation, displays a novel repetitive element and provides racially-dimorphic Taq I RFLPs. Brain Res Mol Brain Res 1992;15:161-6. 
|
| 4. | Bannon MJ, Michelhaugh SK, Wang J, Sacchetti P. The human dopamine transporter gene: gene organization, transcriptional regulation, and potential involvement in neuropsychiatric disorders. Eur Neuropsychopharmacol 2001;11:449-55. 
[PUBMED] |
| 5. | Schultz W. Predictive reward of dopamine neurons. J Neurophysiol 1998;8:1-27. 
|
| 6. | Wheeler DD, Edwards AM, Chapman BM, Ondo JG. A model of the sodium dependence of dopamine uptake in rat striatal synaptosomes. Neurochem Res 1993;18:927-36. 
[PUBMED] |
| 7. | Vaughan RA, Kuhar MJ. Dopamine transporter ligand binding domains. Structural and functional properties revealed by limited proteolysis. J Biol Chem 1996;35:21672- 80. 
|
| 8. | Hersch SM, Yi H, Heilaman CJ, Edwards RH, Levey AI. Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra. J Comp Neurol 1997;388:211-27. 
|
| 9. | Jacobson LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, et al. Prediction of dopamine transporter binding availability by genotype: a preliminary report. Am J Psychiatry 2000;157:1700-3. 
|
| 10. | Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol Sci 1993;14:43-9. 
[PUBMED] |
| 11. | Sokina T, Doolen S, Galperin E, Zahniser NR, Sorkin A. Oligomerization of dopamine transporter visualized in living cells by fluorescence resonance energy transfer microscopy. J Biol Chem 2003;278:28274-83. 
|
| 12. | Kilty JE, Lorang D, Amara SG. Cloning and expression of cocaine-sensitive rat dopamine transporter. Science 1991;254:578-9. 
[PUBMED] |
| 13. | Giros B, el Mestikawy S, Godinot N, Zheng K, Han H, Yang-Feng T, et al. Cloning, pharmacological characterization, and chromosome assignment of the human dopamine transporter. Mol Pharmacol 1992;42:383-90. 
[PUBMED] |
| 14. | Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996;379:606-12. 
[PUBMED] |
| 15. | Seeman P. Nature. Proc National Acad Sci 1975;72:717-14. 
[PUBMED] |
| 16. | Meisenzahl EM, Schmitt GJ, Scheuereeker J, Möller HJ. The role of dopamine for the pathophysiology of schizophrenia. Int Rev Psychiatry 2007;19:337-45. 
|
| 17. | Dao-Castellana MH, Paillère-Martinot ML, Hantraye P, Attar-Lévy D, Rémy P, Crouzel C, et al. Presynaptic dopaminergic function in striatum of schizophrenic patients. Schizophr Res 1997;23:167-74. 
|
| 18. | Schmauss C, Emrich HM. Dopamine and the action of the opiates: A reevaluation of the dopamine hypothesis of schizophrenia. With special consideration of the role of endogenous opioids in the pathogenesis of schizophrenia. Biol Psychiatry 1985;20:1211-31. 
[PUBMED] |
| 19. | Castle D, Wessely S, Der G, Murray RM. The incidence of operationally defined schizophrenia in camberwell. Br J Psychiatry 1991;159:790-4. 
[PUBMED] |
| 20. | Khodayari N, Garshasbi M, Fadai F, Rahimi A, Hafizi L, Ebrahimi A, et al. Association of the dopamine transporter gene (DAT1) core promoter polymorphism A-67T variant with schizophrenia. Am J Med Genet B Neuropsychiatr Genet 2004;129B:10-2. 
[PUBMED] |
| 21. | Rubie C, Scmidt F, Knapp M, Sprandel J, Wiegand C, Meyer J, et al. The human dopamine transporter gene: the 5´-flanking region reveals five diallelic polymorphic sites in a Caucasian population sample. Neurosci Lett 2001;297:125-8. 
|
| 22. | Stober G, Sprandel J, Jabs B, Pfuhlmann B, Möller-Ehrlich K, Knapp M. Family-based study of markers at the 5´-flanking region of the human dopamine transporter gene reveals potential association with schizophrenia. Eur Arch Psychiatry Clin Neurosci 2006;256:422-7. 
|
[Table 1], [Table 2]
|






