|
 
 |
| CASE REPORT |
|
| Year : 2016 | Volume
: 17
| Issue : 1 | Page : 17-19 |
|
Fibrous dysplasia of the humerus: An uncommon cause of pathological fracture in a 56-year-old
Agu Thaddeus Chika1, Ikeanusi Mary Philomena2
1 Department of Surgery, Imo State University, Owerri, Nigeria
2 Department of Surgery, Immaculate Heart Hospital, Nkpor, Anambra, Nigeria
| Date of Web Publication | 16-May-2016 |
Correspondence Address:
Agu Thaddeus Chika
Department of Surgery, Imo State University, Owerri, Imo
Nigeria
 Source of Support: None, Conflict of Interest: None  | Check |
DOI: 10.4103/1595-1103.182479

Fibrous dysplasia is a bone disease that manifests usually before the end of the third decade. Fracture occurring after a minor impact is usually on a background of diseased bone. This case illustrates an asymptomatic bone disease that was diagnosed incidentally when there was failure of union of a humeral fracture after 10 weeks of adequate conservative treatment. Keywords: Fibrous dysplasia, humerus, pathological fracture
How to cite this article:
Chika AT, Philomena IM. Fibrous dysplasia of the humerus: An uncommon cause of pathological fracture in a 56-year-old. Niger J Surg Res 2016;17:17-9 |
How to cite this URL:
Chika AT, Philomena IM. Fibrous dysplasia of the humerus: An uncommon cause of pathological fracture in a 56-year-old. Niger J Surg Res [serial online] 2016 [cited 2018 Jul 21];17:17-9. Available from: http://www.njsrjournal.org/text.asp?2016/17/1/17/182479 |
| Introduction | |  |
Fibrous dysplasia is an uncommon cause of pathological fracture in a 56-year-old. This bone malformation occurs mostly before the end of the third decade.[1] Majority of fibrous dysplasia occurs as craniofacial type, and a characteristic Shepherd Crook's deformity of the proximal femur from fibrous dysplasia is well reported in the literature.[2],[3],[4] Involvement of the upper limb is rarer.[1] Sometimes, fibrous dysplasia is associated with skin hyperpigmentations such as café au lait spots and endocrine abnormalities such as thyroid dysfunction and acromegaly.[4] This skeletal condition presents a diagnostic difficulty especially when these telltale features are absent in patients. Therefore, the failure to associate fibrous dysplasia with the fracture in this patient on the first encounter is an understandable misdiagnosis. This case illustrates the need to review any fracture that fails to heal with adequate conservative treatment when it met all the criteria necessary for union. To achieve union, surgical option of fracture treatment should be explored, and efforts should also be geared toward making a definitive diagnosis.
| Case Report | |  |
A 56-year-old female presented on July 10, 2015, with close fracture of the right humerus following a motorcycle accident. She was alighting from the commercial motorcycle when she slipped and fell on an outstretched hand. A clinical diagnosis of humeral fracture after a fall was made. The X-ray showed a mildly displaced fracture of the midshaft of the humerus [Figure 1]. The entire bony architecture looked normal. The plan was to manipulate and apply cast under anesthesia. After informed consent, this procedure was carried out. The check X-ray showed acceptable alignment and good cortical contact. After 6 weeks, there was no significant callus, and this raised a red flag. The cast was continued for another 4 weeks, and again, there was no clinical or radiological union. The check X-ray showed osteolysis at the fracture ends, but the other parts have equal cortical density. There was no cortical thinning and no cortical expansion. The history of trivial fall, failure of union, and osteolysis at the fracture site raised the suspicion of pathological fracture. Computerized tomography (CT) scan and magnetic resonance imaging were not affordable. Bone scintigraphy would have also been necessary at this point to determine the multiplicity or otherwise of the lesion, but again this facility was not available. The patient was worked up for surgery. The hemoglobin concentration was 12.5 g/dl and serum electrolyte urea and creatinine was normal. Open reduction and plating were then planned, and informed consent was obtained. Intra-operatively, the fracture ends were rather soft and osteoporotic. A bone biopsy was taken from the fracture ends and after a good curettage and satisfactory reduction, a narrow eight-hole Association of Osteosynthesis Dynamic Compression Plate (AO-DCP) was fixed with 6 cortical screws. Patient's recovery was uneventful, and she was discharged after 8 days. Histopathology report showed fibrous stroma in a matrix of trabecular immature bone without differentiated osteoblasts-typical of fibrous dysplasia. By the end of the ninth week, a check radiograph showed that the fracture had united enough for her to engage in basic activity of daily living [Figure 2] and the wound had also healed satisfactorily [Figure 3].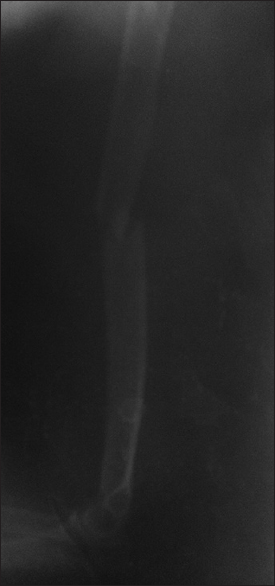 | Figure 1: Plain radiograph showing minimally displaced fracture of the mid--shaft of the humerus, note even cortical thickening and no other evidence of bone lesion
Click here to view |
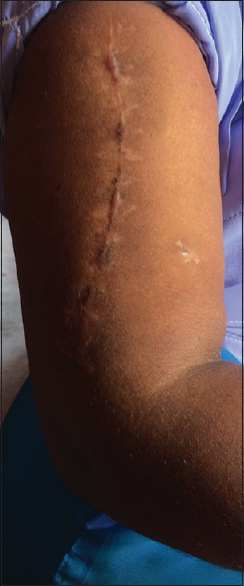 | Figure 3: Nine weeks post operation, healed wound and clinical fracture union, return to activities of daily living
Click here to view |
| Discussion | |  |
Fibrous dysplasia is a rare congenital bone malformation that occurs as craniofacial and skeletal disorder. It is rare in the long bone, and the femur is mostly affected followed by tibia then the upper limb while only craniofacial type constitutes 50%.[2] It is also more common in the first three decades of life which constitute 75% of all fibrous dysplasia, and there is no gender predilection.[1] Therefore, it is not usual to make a diagnosis of pathological fracture due to fibrous dysplasia in a 56-year-old female on first assessment. More so, the initial X-ray did not indicate any underlying lesion bearing in mind that there are typical radiographic features of fibrous dysplasia which include cortical thinning, absent periosteal reaction, and ground glass appearance.[1],[4] These features are pathognomonic, and there may be no need for CT scan in very clear cases.[5] Fibrous dysplasia may be associated with aneurysmal bone cyst in the same lesion, and this necessarily will present a diagnostic difficulty.[6] Though treatment is essentially the same, there is a very small chance of malignant change in fibrous dysplasia, 0.5% of monostotic type [4] unlike in ABC where it has never been reported to occur de novo.
Fibrous dysplasia is largely symptomless and is usually an incidental diagnosis. Some are only diagnosed like in this case, after a pathological fracture. In a small number of cases, there are preceding bone pain and deformity before pathological fracture.[3],[4] Treatment is not necessary if there are no symptoms. Bisphosphonates are indicated for pain, but they do not reduce the incidence of pathological fracture.[7] Intralesional corticosteroid injection and curettage combined with allograft bone grafting are other options of treatment.[8] Impending fracture can be treated this way or by prophylactic hardware fixation.[8],[9]
Occasionally, some skin pigmentations or thyroid dysfunctions may be pointers to this rare bone dysplasia. Our patient did not have any of these associates, and that made the diagnosis even more difficult. Hence, the only reason we taught of a pathological fracture was when there was failure of union despite enough period of adequate fracture immobilization. Moreover, the diagnosis was only certain after biopsy. Histopathological diagnosis may not be adequate in distinguishing fibrous dysplasia from ossifying fibroma which is a very close differential. Whether these bone lesions are extragnathic or gnathic, treatment is essentially the same and the only way of differentiating them is by immunological and/or molecular analysis.[10]
Monostotic fibrous dysplasia is the most common subgroup. It is characterized by a localized lesion involving one bone, and it constitute about 70–80%.[1] This type becomes inactive after skeletal maturity. Polyostotic type, with the involvement of many bones at the same time, is rarer, more symptomatic and can progress into adulthood. Multiple lesions in the same bone are very rare, but if they occur, their influence on the choice of treatment is significant. These multiple lesions may be subtle and may not be detected by plain radiograph. However, most large lesions with cortical thinning and expansion that herald impending pathological fractures will be obvious on plain radiograph.[2],[11] When these multiple lesions exist in addition to an index pathological fracture in the same bone, the use of intramedullary fixation method preferably interlocking nailing becomes necessary to provide both therapeutic and prophylactic fixation of the bone. A CT scan or a radionuclide imaging is useful in making diagnosis of multiple subtle lesions. However, if plain X-ray cannot show these lesions, it may well be that they are not big enough to cause cortical thinning and impending fracture that would warrant prophylactic fixation [2] in which case plating would be suitable. These options of treatment could be with or without bone grafting. Plating is done for a single lesion of the bone that has a good bone stock proximally and distally so as to provide a stable fixation. The patient's X-ray, when reviewed, showed a single area of osteolysis in the humerus and uniformly thick cortical density on the other parts of the entire length signifying that the site of the fracture was the only site of the lesion as obtainable in most monostotic fibrous dysplasia. Consequently, the choice of plating was on the ground of our preoperative plan and because of non-availability of facilities for locked intramedullary nailing of the humerus in our center. Where the facilities are available, the use of intramedullary device would have been preferable to provide both therapeutic as well as prophylactic fixation [9] despite the plain radiograph showing a single area of osteolysis and cortical thinning. This is because there may be subtle lesions that may enlarge over time. However, follow-up X-ray will be necessary to detect any other growth or recurrence.[3] There was clinical as well as radiological union by the 9th week following surgery and patient was able to return to her basic activities of daily living. Satisfactory healing following hardware fixation with or without bone grafting for pathological fractures resulting from fibrous dysplasia of long bones had also been reported by some other authors.[5],[12]
| Conclusion | |  |
Fibrous dysplasia is a rare benign bone lesion and even rarer in a patient in her sixth decade of life. Most are asymptomatic, and they may present for the 1st time as pathological fractures. When adequate conservative treatment fails to heal a fracture that met all the criteria for union, a surgical alternative should be explored. This case and other similar pathological fractures from fibrous dysplasia united following hardware fixation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| References | |  |
| 1. | Chong VF, Khoo JB, Fan YF. Fibrous dysplasia involving the base of the skull. AJR Am J Roentgenol 2002;178:717-20.  |
| 2. | Fitzpatrick KA, Taljanovic MS, Speer DP, Graham AR, Jacobson JA, Barnes GR, et al. Imaging findings of fibrous dysplasia with histopathologic and intraoperative correlation. AJR Am J Roentgenol 2004;182:1389-98.  |
| 3. | DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am 2005;87:1848-64.  |
| 4. | Agarwal M, Balaji N, Sumathi MK, Sunitha JD, Dawar G, Neelakshi SR. Fibrous dysplasia: A review. TMU J Dent 2014;1:25-9.  |
| 5. | Stanton RP, Ippolito E, Springfield D, Lindaman L, Wientroub S, Leet A. The surgical management of fibrous dysplasia of bone. Orphanet J Rare Dis 2012;7 Suppl 1:S1.  |
| 6. | Buraczewski J, Dabska M. Pathogenesis of aneurysmal bone cyst. Relationship between the aneurysmal bone cyst and fibrous dysplasia of bone. Cancer 1971;28:597-604.  |
| 7. | Chapurlat RD, Hugueny P, Delmas PD, Meunier PJ. Treatment of fibrous dysplasia of bone with intravenous pamidronate: Long-term effectiveness and evaluation of predictors of response to treatment. Bone 2004;35:235-42.  |
| 8. | Kelly MH, Brillante B, Collins MT. Pain in fibrous dysplasia of bone: Age-related changes and the anatomical distribution of skeletal lesions. Osteoporos Int 2008;19:57-63.  |
| 9. | Stanton RP, Diamond L. Surgical management of fibrous dysplasia in McCune-Albright syndrome. Pediatr Endocrinol Rev 2007;4 Suppl 4:446-52.  |
| 10. | Toyosawa S, Yuki M, Kishino M, Ogawa Y, Ueda T, Murakami S, et al. Ossifying fibroma vs fibrous dysplasia of the jaw: Molecular and immunological characterization. Mod Pathol 2007;20:389-96.  |
| 11. | Wirbel R, Maier M, Mutschler W, Marzi I. Pathological fracture in osteofibrous dysplasia. Unfallchirurg 2001;104:456-8.  |
| 12. | Vipan K, Badole CM, Wandile K. Management of a case of fibrous dysplasia with pathological fracture of proximal femur. J MGIMS 2013;18:52-4.  |
[Figure 1], [Figure 2], [Figure 3]
|